The performance of CVD coatings can be affected by the base material that they are placed upon. The team at SilcoTek recently tested the compatibility of a range of materials and metals commonly used in analytical testing.
About Silicon CVD Coating Material Compatibility
While it is possible to easily coat most metals, some like nickel are more problematic. It would be inaccurate to suggest no nickel-based metals are suitable for coating, as excellent results can be achieved with Inconel (which is over 70% nickel) and Hastelloy® (which is up to 74% nickel). However, Monel (which is 2/3 nickel and 1/3 copper) is not treatable in this way. These variations in material’s compatibility for CVD coatings can be better understood by exploring alloys and how they react to CVD coatings.
About Alloys and Why They Make a Difference in Coating Results
An alloy is a mix of a particular base element metal with one or more other elements. Because of their often varied compositions, alloys can have very different properties to their base metals.

This means that metals can be combined with other elements to bring out more favourable qualities – for example, aluminum and copper are soft metals, but by combining the two it is possible to create an aluminum alloy that is stronger. Also, iron mixed with a small amount of carbon can create steel, thus providing a number of benefits including greater strength. Furthermore, adding chromium into this mix results in stainless steel, coupling increased strength with improved corrosion resistance.
Alloys can have benefits and drawbacks, both of which can affect coatability. The following section uses nickel as an example to explore this.
How Material Compatibility Can Impact Performance
The CVD coating process is incompatible with pure nickel because the nickel induces a different growth mode in the coating, failing to produce the typical amorphous (non-crystalline or unstructured) coating that can be seen on a stainless steel substrate.
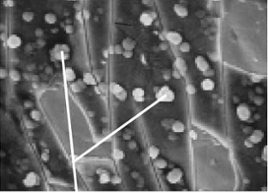
Silicon can bond and diffuse into a wide range of metals, glass or ceramic surfaces but this is not the case with nickel. Once the first few atoms of silicon have bonded to the nickel surface, the remaining atoms will bond to themselves rather than the nickel. If this reaction is magnified, it is possible to see columns of silicon with deep voids, reminiscent of skyscrapers in a large city.
Gaps in the coating when it is applied to nickel mean that chemicals being tested can enter the metal’s exposed surfaces. These surfaces may also become corroded. A general example of magnified columnar growth can be seen in the SEM image below.

When SilcoTek’s amorphous coating is applied to stainless steel, glass, ceramics or many other metal alloys it will uniformly bond to the surface to build a strong coating, layer by layer, over the entire surface of the material in question. This can see seen in the image below.
This Auger plot (below) illustrates how SilcoTek’s silicon barrier coatings bond to the surface of the material they are applied to. Auger electron spectroscopy (AES) sputter depth profile analysis is able to quantify the material composition and diffusion characteristics of silicon coatings, in this case the SilcoNert® coating.
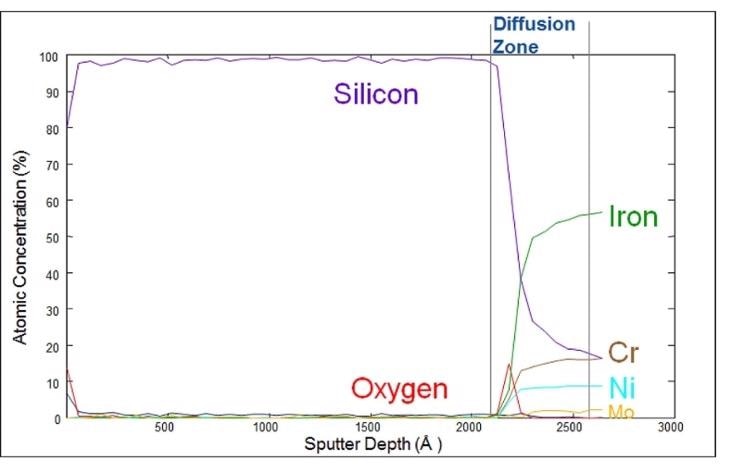
The Auger plot displays a layer of around 2000 angstroms (200 nm) of silicon. There is an approximately 500 angstrom diffusion zone between the surface of the stainless steel and the silicon coating itself. Were an Auger depth profile of a coated nickel part be viewed closely, it would show areas of nickel with little to no coating, whilst others would have a much greater depth profile.
More on Alloys
SilcoTek’s coating can completely coat materials where nickel is alloyed with other stabilizing elements. Cr (chromium), P (phosphorous) or Si (silicon) are examples of elements which can be used to stabilize a nickel-based alloy, and this stabilization can take place even when Ni (nickel) is the major component in the base metal.
Hastelloy®, Inconel or typical nickel brazing filler alloys such as Ni-Cr-P or Ni-Cr-Si-P are common examples of this, and in these cases, the coating can bond to the more compatible alloy because its characteristics allow silicon to preferentially bond to the surface in a uniform way. For this reason, the coating can coat superalloys and Ni brazing material with ease.
Exceptions to the Rule
There are some cases however, where alloying nickel can continue to cause problems with coating the material. For example, if nickel were to be alloyed with copper – another difficult to coat element – there would be no synergy or ‘stabilizing force’ which would allow CVD coatings to bond to the surface. As such, copper nickel alloys - such as Monel - are incompatible with this process because the base elements themselves are already incompatible on their own.

This information has been sourced, reviewed and adapted from materials provided by SilcoTek.
For more information on this source, please visit SilcoTek.