
When looking to improve sensitivity and precision of their instruments, customers often evaluate the coating surface of such instruments for reactivity so as to confirm the coating meets performance parameters. There are various forms of testing a coating for inertness and purity, however, so which one is the most effective? The answer to this shall be discussed here.
It can be difficult testing and evaluating a coating or surface for adsorption, inertness and purity. This process is particularly difficult when testing compounds at trace levels. If you’re struggling to find a method for evaluating a coating or surface then you’re in luck! SilcoTek have over 30 years of experience recommending the processes or methods available for this kind of evaluation. The following represents just a few tips amassed over the years.
This article shall feature:
- Tips on how to evaluate the inertness or reactivity of a surface
- How the use of different test compounds and concentrations can alter results
- Sources for inertness test design standards and specifications
- Ideas of test system design
Selecting the Compound & Concentration
How can I be sure that the surface I choose is inert in my test? This is a common, and difficult to answer question, often posed in the testing of coatings and surfaces. Some surfaces may perform extremely well for one compound but not others. Furthermore, some surfaces or coatings may perform perfectly well with higher levels of concentration but not for trace analysis or speciation.
Selecting the Compound
If you only have one compound that you expect to be testing then this step is very straightforward; simply select this compound at the required concentration and test. It is important here to remember to run a statistically significant number of trials, perhaps n=5, as well as testing a few coated flow path component samples so as to assure repeatability. Prior to testing it is important to understand the capabilities of the coating or surface in question. Information on some of SilcoTek’s coatings can be found here:
The majority of analytical systems will, however, be exposed to a large number of active compounds which greatly complicates matters. Given that it is not possible to simply test all reactive compounds known to man, one needs to narrow down this field. Instead of spending a lifetime testing various flow path surfaces, one could consider testing instead a class of reactive compounds as opposed to all potential analytes. For example, endrin and DDT are both superb environmental test compounds given that they rapidly degrade when they come into contact with a reactive surface, especially if said surface is hot.
A good reference source for information on chemicals containing target compounds for subsequent analysis is chromatography companies. Whether it is environmental testing, clean air standards, whole air monitoring, or oil and gas sampling, information can be sourced from various companies, many of which offer calibration samples. These samples can become expensive quickly, however, and therefore when testing multiple compounds one must be prudent in your selection.
Here are some links to relevant company information on sourcing chemical standards:

Concentration Matters
Unfortunately selecting the test compound is not the only step here; one must also select the compound concentration. The compound concentration is, in many ways, every bit as important as the test compound. To demonstrate this point think of sulfur analysis. A stainless steel surface may yield perfectly fine results at percent level analysis but sample at part per million levels and it's a completely different story.
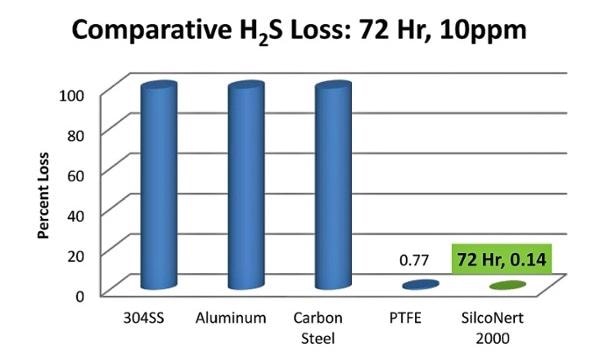
Sulfur is known to adsorb or adhere to stainless, glass or ceramic flow paths. However, if high concentration sulfur is put through the flow path then the active surface will be seasoned, or primed, to the extent that tests will yield seemingly good results. Despite this, at ppm or lower levels, sulfur will be absorbed onto the surface due to the lack of the analyte causing no priming to occur. This causes almost all the sample to be lost. Here is a chart comparing the loss rate of trace H2S which demonstrates that almost all H2S is lost within an hour of exposure. The SilcoNert coated surface adsorbs virtually no sulfur compound:
https://www.silcotek.com/blog/how-to-test-an-inert-coating-for-inertness-and-purity
Important factors to think about when selecting concertation:
- Regulator requirements
- Industry/customer standards
- ASTM recommended methods
- Internal specifications
The lower concentration limits selected for use during a surface or coating test may be dependent on the lower calibration point of the system or instrument in use. This can often be found by looking at the relevant regulatory or industry standards which dictate a calibration sample range. It is often then crucial to test a system for levels slightly above and below this range. For example, a calibration run for a flare CEMS may require a total sulfur sample be run at a lower limit of 5 ppm and a higher limit of 200 ppm for an operation range of 10 to 150 ppm. This upper limit of 200 ppm may be easy to achieve, the lower limit of 5ppm on the other hand can cause all sorts of problems. Some such problems generated when testing at lower limits include, but are not limited to:
- Faster expiration of the standard or stability of the chemical standard
- Contamination and cross contamination
- Adsorption by an uncoated reactive fitting or fritted filter
- Seal material like o-rings that may be reactive
- Moisture contamination may cause adsorption
- Corrosion related particulate adsorption
- Seams or pinholes that promote cross contamination or trace sample loss
- Cleanliness of the system
- System surface temperature, phase change
- Detector or instrument sensitivity to the target compound
When testing reactive samples at low levels the finer details make a big difference. Take a note of system parameters and any changes made during the test to assure an apples to apples comparison and to avoid "getting lost in changes" while troubleshooting a system.
System Design, Testing an Inert Coating
Even now that you have selected the samples, selected the sample concentration and found the test methods based upon the industry, regulatory or company procedures, you are still not quite ready to begin your tests. You must first select the test system itself. The following represent a few tips to ensure the generation of reliable, accurate test results.
The test system may be set up in accordance with regulatory or industry practices; when evaluating a surface always take into consideration the following:
- Apples to apples. Don't change the test conditions during the evaluation
- Nooks and crannies. Minimize surface pin holes, threads grooves or other areas where a sample can get trapped and cross contaminate a test.
- Combining reactive surfaces with inert surfaces. It is not possible to test an inert surface simply by placing it in line with a reactive surface. For trace analysis the sample will be adsorbed by the reactive areas so a comparison of surfaces would yield the same results. Instead, it is necessary to either coat the entire test flow path and compare 2 competing flow paths or select a known inert surface and change only a portion of the flow path for comparison.
- Minimize flow path complexity. In order to minimize test variables keep the flow path as simple as is possible.
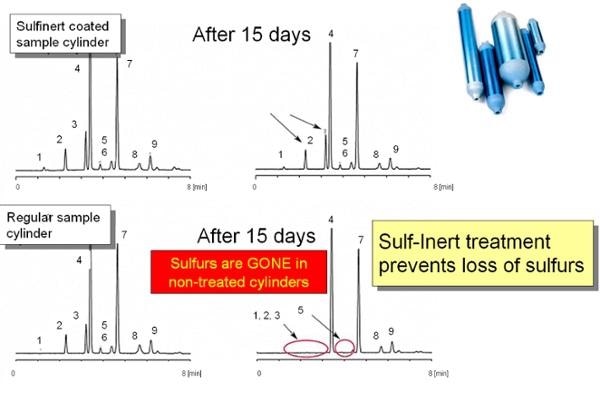
- Make the exposure time adequate to determine effectiveness. Either flow through a long tube at low flow or through a high surface area component like a sintered metal frit to be sure there's adequate surface exposure. Alternatively, test by residence time in a sample cylinder. This method can take a long period of time, up to weeks, but will allow you to establish the degradation rate of the sample. To accelerate the test, consider heating the sample cylinder so as to accelerate the reaction rate. Here's an example of 15 day comparative testing for sulfur adsorption. The top chromatographs continue to show sulfur peaks after 15 days for SilcoNert® (Sulfinert®) coated sample cylinders. Uncoated stainless steel sample cylinders adsorb the sulfur (lower graphs).
- Seals. Viton, septa or other seal surfaces can be active. Minimize the use of polymeric seals to limit adsorption.
- Instrument capability. If your instrument is not capable of testing to the desired concentration, or it is not set up to yield reliable results for a target compound then it is not worth evaluating the surface. Instrument factors to consider are:
- Detector (designed for the target compound)
- Appropriate instrument (LC or GC?) Should be capable of testing in the target sensitivity range for those compounds.
- Calibration. Instrument should be calibrated and maintained
- Training. The analyst should be familiar with the instrument and detector.
Take a Short Cut
Take advantage of the work already done by others. There already exist a large number of papers available in which comparisons have been drawn for the inertness of various samples. SilcoTek house a vast library of comparative papers demonstrating the effectiveness of their coatings in inertness applications. It isn’t necessary to simply take their words for it, or to just use someone else’s test results for your own application, however reading the results of another's tests can be an excellent starting point in generating ideas for testing relevant to your own applications and understanding the limitations of a surface.

This information has been sourced, reviewed and adapted from materials provided by SilcoTek.
For more information on this source, please visit SilcoTek.