Metal-organic frameworks, also known as MOFs, are based on coordination bonds, which are usually less stable when compared to covalently bonded materials.1-4 Hence, it is a critical challenge to overcome the stability of MOFs.5-15 Stronger MOFs are resistant to moisture, have a high gas storage capacity, and also have an ability to be scaled up easily. These properties make them more appropriate for commercial sale. However, the manufacturing process of these MOFs presents a major problem because these materials were produced using “one-pot” synthesis which cannot be easily controlled.16
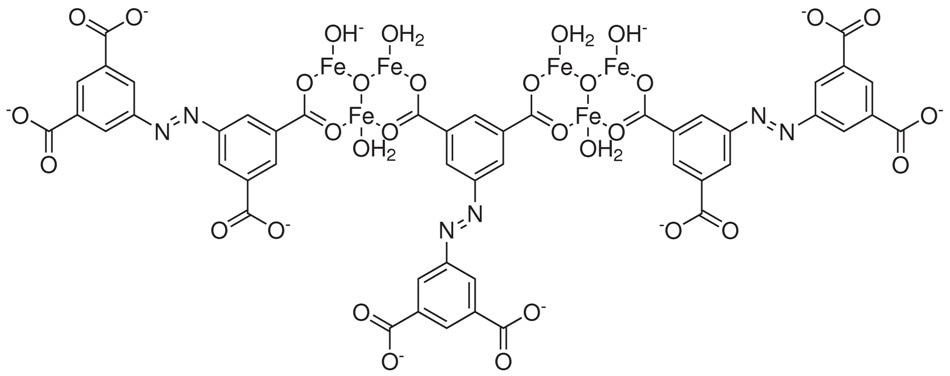
In order to mitigate this challenge, a new method based on thermodynamic and kinetic control of MOF crystallization has been applied to produce Fe-MOF single crystals with pre-synthesized metal building blocks [Fe2M(μ3-O)(CH3COO)6] (M=Fe2+,3+, Co2+, Ni2+, Mn2+, Zn2+). Among the MOFs synthesized was the PCN-250, which has demonstrated a high total CH4 uptake of 200 V STP/V at 35 bar and 298 K and a high total H2 volumetric uptake of 60 g L-1 at 40 bar and 77 K.17,18
High Volumetric Gas Uptake
The structure of the PCN-250 is responsible for its high methane uptake and represents a key feature of this material.18 A performance plot of PCN-250’s deliverable capacities is shown in Figure 1. PCN-250 can serve as an excellent absorbent for methane storage, provided a pressure range between 1 and 35 bar is used with the highest methane loading at 35 bar (298 K and 200 v/v). Due to the high crystal density of the MOFs, PCN-250 has a record-high H2 uptake of 28 g L-1 and 3.07 wt% at 1.2 bar and 77 K and also a total volumetric H2 uptake of 60 g L-1 at high pressure.
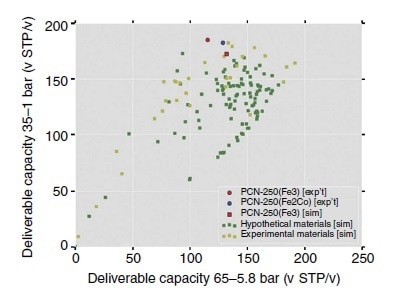
Figure 1. Performance plot of deliverable capacities using two different pressure swings. Shown are the predictions for the Fe-MOFs synthesized here (yellow) and in silico-predicted structures (green). Red and blue points show PCN-250.
Figure 2 shows how each side of the cubes in PCN-250 is covered with charged open metal sites by the ligand, with the sites stationed around the channels between each cube. This enables the interspace to be available for adsorption of gas, which in the PCN-250 product has a strong interaction with both CH4 and H2 molecules. Additionally, a highly efficient utilization of space for increased uptake of volumetric gas can be realized by inducing polarization of gas molecules via charge-induced dipole interaction.
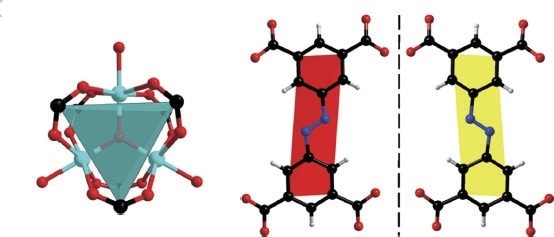
Figure 2. Structures of PCN-250
Chemical Stability of PCN-250
Studies have demonstrated that PCN-250’s chemical stability remains intact even if a single iron atom is replaced for a softer Lewis acid metal, M(II), in the μ3-oxo cluster. The PCN-250 continues to be intact in different pH values ranging from 1 to 11 over a 24-hour period. It was also observed that PCN-250 continued to be strong in H2O after a period of 6 months in neutral conditions. Following multiple pH treatments, no major change was observed in the N2 adsorption isotherms of the material, causing researchers to conclude that phase transition or MOF decomposition did not take place.
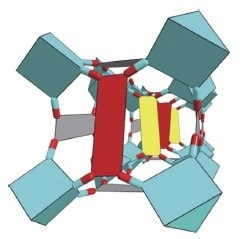
Figure 3. Structure drawing of PCN-250
The high gas uptake and excellent scalability and stability of the PCN-250 make it a propitious material for storing natural gas for a variety of applications such as gas purification and power systems.
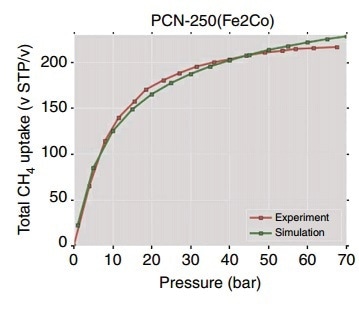
Figure 4. Total CH4 adsorption uptake of PCN-250 at 298 K
Key Properties
- Form and color: dark red-brown powder
- Stable in aqueous and water solutions
- Excellent uptake of methane and hydrogen
References and Further Reading
- Zhou, H.-C., Yaghi, O. M. & Long, J. R. Introduction to metal–organic frameworks. Chem. Rev. 112, 673–674 (2012).
- Fe´rey, G. et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 309, 2040–2042 (2005).
- Serre, C. et al. Role of solvent–host interactions that lead to very large swelling of hybrid frameworks. Science 315, 1828–1831 (2007).
- Banerjee, R. et al. High-throughput synthesis of zeoliticimidazolate frameworks and application to CO2 capture. Science 319, 939–943 (2008).
- Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
- Herm, Z. R. et al. Separation of hexane isomers in a metal-organic framework with triangular channels. Science 340, 960–964 (2013).
- Deng, H. et al. Large-pore apertures in a series of metal-organic frameworks. Science 336, 1021–1023 (2012).
- Bloch, E. D. et al. Hydrocarbon separations in a metal-organic framework with open iron (II) coordination sites. Science 335, 1606–1610 (2012).
- Farha, O. K. et al. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2, 944–948 (2010).
- Cavka, J. H. et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 130, 13850– 13851 (2008).
- Fe´rey, G. et al. A hybrid solid with giant pores prepared by a combination of targeted chemistry, simulation, and powder diffraction. Angew. Chem. Int. Ed. 43, 6296–6301 (2004).
- Surble´, S. et al. A new isoreticular class of metal-organic-frameworks with the MIL-88 topology. Chem. Commun. 3, 284–286 (2006).
- Dincă, M. et al. Hydrogen storage in a microporous metal–organic framework with exposed Mn2t coordination sites. J. Am. Chem. Soc. 128, 16876–16883 (2006).
- Li, J.-R., Sculley, J. & Zhou, H.-C. Metal–organic frameworks for separations. Chem. Rev. 112, 869–932 (2012).
- Yoon, M., Srirambalaji, R. & Kim, K. Homochiral metal–organic frameworks for asymmetric heterogeneous catalysis. Chem. Rev. 112, 1196–1231 (2012).
- Tranchemontagne, D. J., Mendoza-Corte’s, J. L., O’Keeffe, M. &Yaghi, O. M. Secondary building units, nets and bonding in the chemistry of metal–organic frameworks. Chem. Soc. Rev. 38, 1257–1283 (2009).
- Murray, L. J., Dincă, M. & Long, J. R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 38, 1294–1314 (2009).
- Makal, T. A., Li, J.-R., Lu, W. & Zhou, H.-C. Methane storage in advanced porous materials. Chem. Soc. Rev. 41, 7761–7779 (2012).
- Schoedel, A. & Zaworotko, M. J. [Μ3(μ3-O)(O2CR)6] and related trigonal prisms: versatile molecular building blocks for crystal engineering of metal–organic material platforms. Chem. Sci. 5, 1269–1282 (2014).
- Liu, Y. et al. Assembly of metal–organic frameworks (MOFs) based on indium trimer building blocks: a porous MOF with soc topology and high hydrogen storage. Angew. Chem. Int. Ed. 46, 3278–3283 (2007).

This information has been sourced, reviewed and adapted from materials provided by Strem Chemicals.
For more information on this source, please visit Strem Chemicals.