Novel materials for Li-ion batteries are currently being researched and developed with the goal of increasing the lifetime and capacity of batteries. Whilst SEM is an important tool in this research, determining how lithium is distributed in the sample remains a key challenge.
Detecting K-band X-rays (which indicate lithium) from metallic lithium samples was first demonstrated by Oxford Instruments, Burgess et al.1, and of a lithium compound by Hovington et al.2
This article will cover recent progress in the characterization of lithium-ion batteries using SEM equipped with EDS, and will explore how the Extreme detector can be used to study the lithiation of graphite anodes, and how the conventional detector can be used to study ceramics that contain lithium.
Li Detection by EDS
The Ultim® Extreme EDS detector was used to measure graphite anodes. Following charging the graphite particles contain lithium intercalated between the layers of the graphene, and lithium-based compounds also form on the surface, firstly as a solid- electrolyte interphase (SEI) which grows into dendrites following more charge-discharge cycling. For some graphite particles no lithium was detected, whereas other particles showed significant lithium present in their EDS spectra.
Figure 1 shows the same lithiated graphite particle prior to, and following, electron beam exposure. The particulate matter that forms during electron beam exposure can be observed on the surface of the particle (Figure 1b).
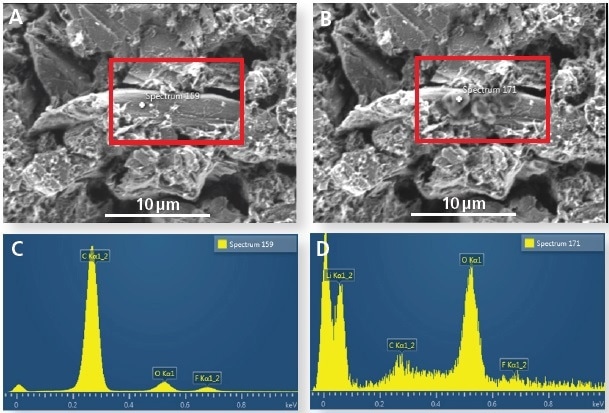
Figure 1. (A) Graphite particle before electron beam irradiation and (B) after irradiation. (C) EDS spectrum before prolonged irradiation (no Li detected) and (D) after irradiation (showing a Li signal).
The EDS spectrum, alongside the information depth for graphite at 3 kV, indicates that significant amounts of lithium are present at the particle’s surface. The origin of this lithium is currently under research, i.e. is it formed from the surface SEI layers, dendrites from cell cycling, lithium in graphite samples, or if the diffusion of lithium depends on the orientation of grains.
A significant conclusion from the data is that lithium is highly mobile under the electron beam and therefore any quantitative measurements should be treated with caution.
Thickness Measurement of Li Layer
Without direct measurement of the lithium X-rays it is possible to estimate the thickness of a hypothetical lithium surface layer using the AZtec® LayerProbe®. To demonstrate this a sintered pellet of Li1.4Al0.4Ge1.6(PO4)3, a solid electrolyte of 0.3 mS/cm conductivity at r.t., was analyzed.
Some of the pellet was in contact with Li foil for a number of days, following which the foil was removed and EDS analysis was carried out.
The EDS spectra of the sample with and without lithium exposure were compared, based on the assumption that contact with foil resulted in a transfer of lithium to the surface to form a lithium rich layer. Comparing the EDS spectra of both samples shows a distinct attenuation in the O K, P, K, Ge, L and Al, K X-ray lines in the spectrum for the region in contact with the Li foil (Figure 2).
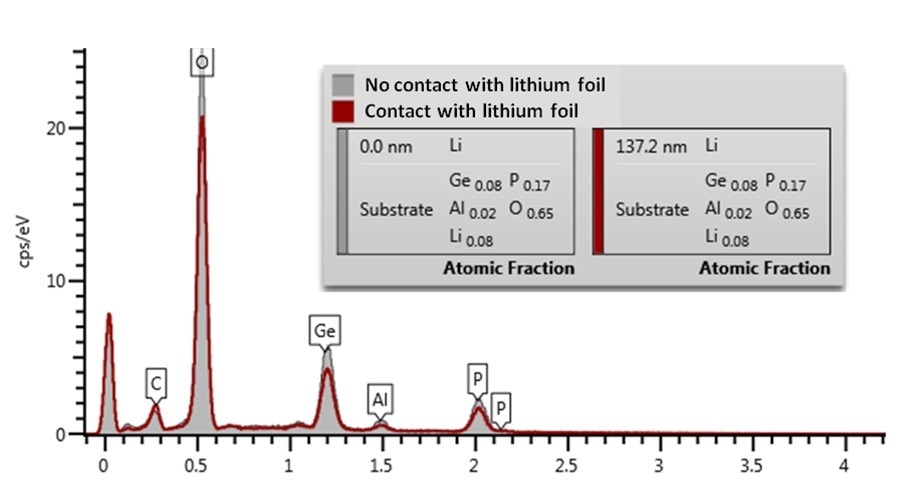
Figure 2. EDS spectra and Li layer thickness estimation from the areas of the sample having been in contact and not in contact with Li.
The carbon peak height for both samples is approximately the same, meaning geometric effects, such as surface tilt or shadowing, can be excluded. The thickness of a hypothetical lithium layer in the Li1.4Al0.4Ge1.6(PO4)3 pellet can be calculated by AZtec LayerProbe using the extent of X-ray attenuation.
Assuming that it is metallic lithium (ρ = 0.53 g/cm3) coating the contacted region of the sample the LayerProbe determined that a lithium layer thickness of 100-150 nm would result in the level of attenuation observed. For comparison, EDS did not determine any attenuation in the O K, P, K, Ge, L and Al, K X-ray lines for regions of the sample which had not been in contact.
Conclusion
The case study explored in this article highlights the power of SEM/EDS in the analysis of Li-ion battery materials. The results demonstrate that Li X-rays can be detected using the Extreme detector, but that these results can be hard to accurately interpret due to lithium’s mobility when exposed to an electron beam.
However, it is also possible to study lithiation processes indirectly, by using the attenuation that a hypothetical surface layer of lithium exerts on the X-ray emissions of other elements in the sample.
References and FUrther Reading
- Burgess S, Li X, Holland J. 2013 Micro Anal 27:S8–S13.
- Hovington P, Timoshevskii V, Burgess S. et al 2016, Scanning, in print.

This information has been sourced, reviewed and adapted from materials provided by Oxford Instruments NanoAnalysis.
For more information on this source, please visit Oxford Instruments NanoAnalysis.