Performing the entire analysis from a single dissolution has emerged as a necessity with the advent of the modern analytical techniques. XRF Scientific has designed multiple models of fusion machines to enhance the creation of solutions.
These instruments have the advantage of increasing repeatability and productivity without the risks for the operators to manipulate dangerous reagent. The processes are straightforward, fast and cleaner.
There are two different types of fusion for ICP that demand a specific environment and specific conditions. Benefiting from over 25 years of experience, XRF Scientific has developed the most adequate fusion machines with remarkable advances to precisely meet the specific requirements of those fusions.
- The Phoenix GO ICP for Peroxide, Carbonate or Hydroxide Fusion

- The xrFuse ICP for Borate Fusion

The Phoenix GO ICP for Peroxide, Carbonate or Hydroxide Fusion
The peroxide fusion involves mixing a powder sample with an alkali fusion flux inside a nickel or zirconium crucible, heating to about 600 – 800 °C with agitation until complete reaction and dissolution of the sample in the flux. The holt mixture is then cooled in situ to produce a vitreous mass which is often colored and easily soluble in diluted acids.
Reactions are made at various temperatures in this type of fusion, and they can sometimes be extremely energetic, enabling a risk of spattering. The Phoenix GO ICP is the optimal tool as it can be programmed with an unlimited amount of heating steps to gently bring the mix at temperature, all being done behind a safety door to protect the operator.
Below is the list of the most used flux and examples of their applications.
| FLUX |
Melting P. (°C) |
Dilution |
Applications |
| NaOH |
318° |
1:5 |
Determination of Mo, W, F, Cl and B.
Fusion of Silicates, Aluminates, Titanium oxide, Bauxites, Phosphates, Fluorides, Carbides, Silicon, Sulfides, |
| KOH |
360° |
1:5 |
Fusion of Tantalum and Niobium ores |
| Na2O2 |
460° |
1:15 |
Determination of Pt, Rh and Pd.
Fusion of Refractories, Chromites, Resistant alloys…
Sodium peroxide is very reactive |
| Na2O2 – Na2CO3 |
- |
1:15 |
By adding 1g of sodium carbonate to 5 or 8g of sodium peroxide, violent reactions are less likely to occur |
| Na2CO3 |
853° |
1:4 |
Determination of F, Cl and B.
Fusion of minerals, Aluminosilicates, Barite,. |
| K2CO3 |
903° |
1:4 |
Determination of F, Cl and B.
Fusion of Aluminosilicates, Zirconium silicate |
| Na2CO3 – K2CO3 |
712° |
1:4 |
Determination of F, Cl and B.
Fusion of Aluminosilicates, Zirconium silicate |
Their reactivities can be classified as follows: 1.Peroxide, 2. Hydroxide, 3. Carbonate
Typical Sample Preparation
- Zirconium crucible (nickel crucible is limited at 600 °C)
- Flux: NaOH
- Dilution: 1:5 (1 g sample for 5 g flux)
- 5 - 10 minutes of heating from 300 °C up at 600 °C
- Heating 1, at 300 °C typically used to pre-heat the sample, no agitation
- Heating 2, at 400 °C typically used to initiate the reaction, little agitation
- Heating 3, at 600 °C typically used to complete the reaction,
- 4 minutes of cooling
- Cooling 1, soft cooling
- Cooling 2, forced cooling
- Dissolution in a 5% HNO3 w/v

Phoenix GO 2000 & 8000 ICP

These new gas fusion machines are designed to do numerous and repeatable fusions with hydroxide, peroxide and carbonate. This new Phoenix machine doesn’t need oxygen and compressed air as it uses gas only.
They are made to endure repetitive exposure to aggressive environments and designed for high up time applications. With 8 fusion stations, the Phoenix GO 8000 ICP is specifically designed for high volume.
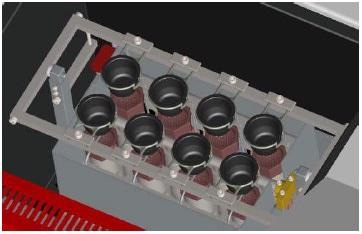
With an infinity of steps configurable, the design of the user interface can meet the needs of the most versatile application.
Key Features
- Gas only fan forced burner – new technology
- Temperature: 300 – 1000 °C
- 2 or 8 fusion stations
- Fully automatic: preheating, heating, cooling and swirling
- Temperature measurement: thermocouple near flame (indicative)
- Multiple heating steps programmable
- Touch screen user interface
- Up to 100 user-defined recipes with naming flexibility
- Crucible cooled above the burner
- Homogenization by swirling
- USB communication link
- Emergency stop button; automatic gas cut-off safety system, active burner monitoring cold-to-cold operation, safety rated lid switch
| Item Code |
Description |
| PHOENIX GO 2000 ICP |
Produces 2 samples simultaneously – fusion for ICP |
| PHOENIX GO 8000 ICP |
Produces 8 samples simultaneously – fusion for ICP |
The xrFuse ICP for Borate Fusion
The borate fusion involves mixing a powder sample with a fusion flux inside a platinum-5% gold crucible, heating to about 1000 – 1150 °C with agitation until complete reaction and dissolution to form a homogeneous molten glass. The molten glass is then casted into the beaker until the vitreous mass is completely dissolved.
This method is the most common for the analysis of elaborate and geological materials. It allows the determination of major elements and a certain number of elements in traces. One major asset of the xrFuse is that they are ready for serials dissolutions, the fusion and digestion are completely automated inside the machine.
Here below is the list of the most used flux and their applications.
| FLUX |
Melting P. (°C) |
Dilution |
Applications |
| Lithium Metaborate |
845° |
1:4 |
Fusion of Silicates, Rocks |
| Lithium Tetraborate |
920° |
1:4 |
Fusion of Dolomites, Carbonates, Aluminates |
| Mix of 66% Lithium Tetraborate and 34% Lithium Metaborate |
875° |
1:4 |
Fusion of Refractories, Chromites, Fusion of Aluminosilicates, Zirconium silicate, Limestone, Lime |
To be sure the transfer is complete, a sufficient amount of releasing agent is used. Generally, 0.5% Lithium Bromide.
Typical Sample Preparation
- Platinum-5% gold crucible
- Flux: Lithium Metaborate + 0.5% LiBr
- Dilution: 1:4 (0.4 g sample for 1.6 g flux)
- 5 - 10 minutes of heating at 1000 °C
- 4 – 8 minutes dissolution in a beaker containing about 90 ml of 5% HNO3 w/v

xrFuse 1, 2 and 6 ICP

Rugged electric fusion machine designed for the preparation of 1 to 6 acid solutions for analysis by AA or ICP.
The fused samples are automatically poured into the Teflon beakers containing the dilute acid solution when working in ICP mode. The beakers remain in position until the complete dissolution with the help of magnetic stirring.

The machine is equipped with an exhaust fan to remove the acid vapor created during the pouring of the hot liquid into the beaker.
The ICP modules can accept 1, 2 or 6 beakers of 100 ml depending on the models. Each position is equipped with separate magnetic stirring.

Key Features
- Maximum temperature 1250 °C (1200 °C continuous)
- Fully automatic: premelting, melting, pouring, shacking and magnetic stirring
- Touch screen user interface, password protected engineer levels
- Up to 12 user-defined recipes with naming flexibility
- Real time temperature reading
- Homogenization by rocking, variable angle and speed
- Two stages magnetic stirring
- Adjustable pouring angle
- Crucible holder: hi-purity ceramic, no contamination
- Emergency stop button
- Cool touch glass viewing window; lid safety-interlocked during fusion and standby mode
- Safe enclosed, cold to cold operation
- Ethernet and USB communication link
| Item Code |
Description |
| xrFuse1 |
Produces 1 sample simultaneously ICP |
| xrFuse2 ICP |
Produces 2 samples simultaneously ICP |
| xrFuse6 ICP |
Produces 6 samples simultaneously ICP |

This information has been sourced, reviewed and adapted from materials provided by XRF Scientific.
For more information on this source, please visit XRF Scientific.