Applications in a wide variety of fields can benefit from the use of pickering emulsions as a result of their strong stability when compared to systems which are stabilized by surfactants. These fields include vesicle and responsive materials, drug delivery, catalytic facilitation, porous materials, and safer formulation in cosmetics.
Pickering emulsions also have numerous beneficial properties – they are more environmentally friendly, with a low carbon footprint, and a lesser degree of irritability.
This article outlines the results from a study of pickering emulsions used in the pharmaceutical field, using an antifungal medication which was stabilized with a biocompatible component. Surfactant free emulsions are particularly useful in the pharmaceutical field in order to avoid protein denaturation, hemolysis, and skin irritation.
Definition
Pickering emulsions are emulsions which are stabilized by solid particles ranging between 50 and 500 nm. These particles adsorb onto the interface between the two phases.
It is possible to tune the emulsion properties in function of the particle affinity with water. Surfactant molecules exist in a dynamic equilibrium at the interface of the oil and water and can desorb or adsorb rapidly. In contrast, particles demonstrate irreversible adsorption.
The stability of particles over extended time periods may also be explained by this strong adsorption of particles at the interface (even with large droplet sizes).
Consequently, when confronted with destabilization phenomena like Ostwald ripening and coalescence, pickering emulsions are extraordinarily stable. They also have the potential to enhance oxidative stability when compared to systems stabilized by surfactants.
Reminder on the Technique
Based on Static Multiple Light Scattering, Turbiscan technology consists of a light source (880 nm) sent onto a sample. Backscattered (BS) and transmitted (T) signal is then acquired over the entire height of the sample.
The instrument facilitates the monitoring of physical stability when this measurement is repeated over time at varying frequencies. According to the Mie theory knowing refractive index of continuous (nf) and dispersed phase (np), the signal is directly linked to the particle concentration (φ) and size (d): BS=f(φ, d, np, nf).
Method: Pharmaceutical Application
Clays, silica, titanium oxide, latex, and calcium carbonate are the most commonly used pickering particles. The focus of this study is biocompatible native cyclodextrins (CD) for antifungal medication. This is based on econazole nitrate salt for the treatment of skin infections. As shown in the table below, varying formulations have been tested which alter the oil phase and type of the cyclodextrin.
Table 1. Composition of 6 formulations of fungal Pickering emulsion
| Formulation |
Stabilizer |
Oil |
F1
F2
F3 |
α-CD
β-CD
γ-CD |
Liquid paraffin |
F4
F5
F6 |
α-CD
β-CD
γ-CD |
Isopropyl myristate |
Results
The graphs featured in Figure 1 show the delta-backscattering signal for the emulsions F1 and F4 against the sample height in function of time, from the blue to the red curves.
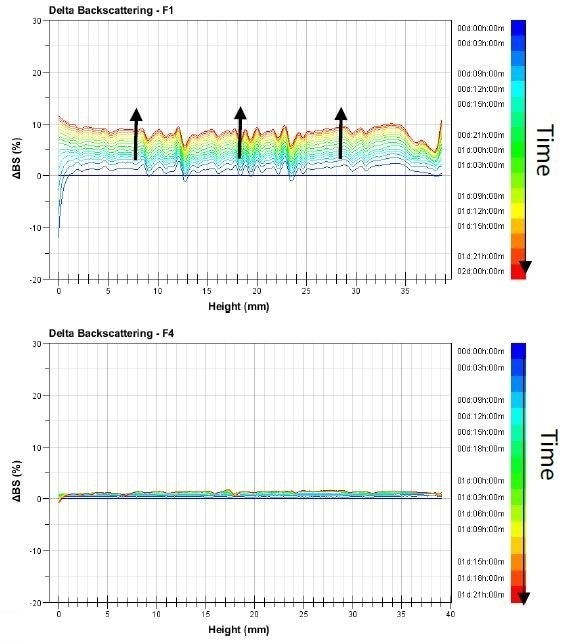
Figure 1. Backscattering evolution versus time and sample height of Pickering emulsion F1 and F4 analyzed at 25 °C.
All of the samples’ Turbiscan profiles show an evolution over the sample’s entire height. This means that the size of the droplets is increasing. From one sample to another, the intensity of the variations changes. To put it another way, with varying intensities in the samples, coalescence occurs.
Courtesy of the Turbiscan Stability Index (TSI), monitoring and quantifying the destabilization kinetics is possible. This is calculated by the addition of every signal variation which is detected as a result of destabilization phenomena (clarification, sedimentation, size variation etc). At any given ageing time, the higher the TSI value, the shorter the sample’s stability.
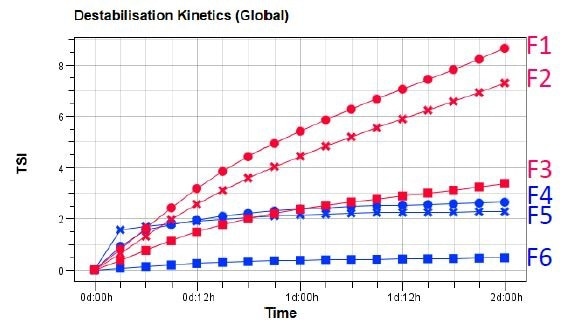
Figure 2. TSI evolution versus time of Pickering emulsions F1-F6.
The results enable the ranking of formulations in terms of stability: emulsions F1-F3 which contain paraffin oil are less stable than F4-F6, which contain isopropyl myristate oil. The latter oil can establish O-H ---O hydrogen bonds with CD cavity in order to achieve higher binding constants than with myristate.
A comparison of the effect of CD particles demonstrates that emulsions with β-CD and α-CD are less stable than those with γ-CD. This is likely due to the fact that the inclusion complex based on γ-CD is larger, enabling the binding of complexes together for a denser coating.
Conclusion
Turbiscan experiments have been performed in order to compare and quantify the emulsion stabilities of varying formulations by altering the continuous phase and the size of the cyclodextrin. Without needing to add charges or polymers, the coalescence is limited by the surface coating of oil/cyclodextrins inclusion complexes.
The global physical stability ranking can be realized in one click, and from these measurements the interface coverage of particles can be deduced.
Reference
- L. Leclercq, V. Nardello-Rataj, Pickering emulsions based on cyclodextrins : A smart solution for antifungal azole derivatives topical delivery. European Journal of Pharmaceutical Sciences 82 (2016) 126-137.

This information has been sourced, reviewed and adapted from materials provided by Microtrac.
For more information on this source, please visit Microtrac.