In pharmaceutical production, excipients are essential in most dosage forms such as granules, powders, (film) tablets, inhalants, ointments, dragées, liquids or gels. They enable or improve the production of safe-to-use and simple dosage forms, ensure sufficient durability and control the active ingredient release and bioavailability.
With regards to the evidence of safety, efficacy and quality, they have equal status with the active ingredients according to regulatory authority requirements. Often, both particle shape and particle size are decisive to the quality.
Methyl cellulose is a cellulose derivative and a polysaccharide. It is present as a hydrophilic white powder with a fibrous structure, is easily digestible and soluble in cold water. Functionally, it controls the release of the active ingredient as a film former by embedding active ingredients or cores as a film tablet. It serves as a water-soluble binder or as a thickening and gelling agent in wet granulation and as a dry binder to improve compressibility in the tableting process. The functionality of the methyl cellulose is determined by the thickness of the fibers.
Particle Size Distribution (LEFI) and Particle Shape Distribution (Elongation)
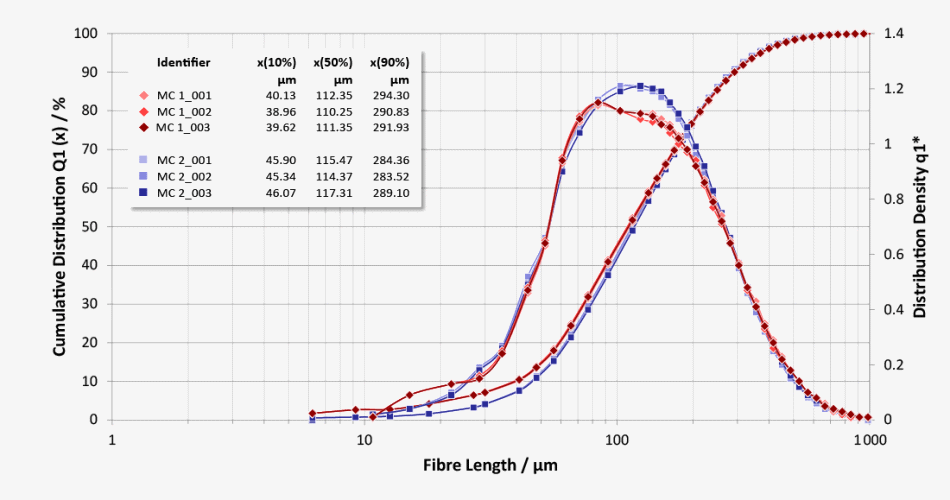
Q1 length distribution shows minor differences between two batches of methylcellulose (evaluation according to the length of the fibers (LEFI))
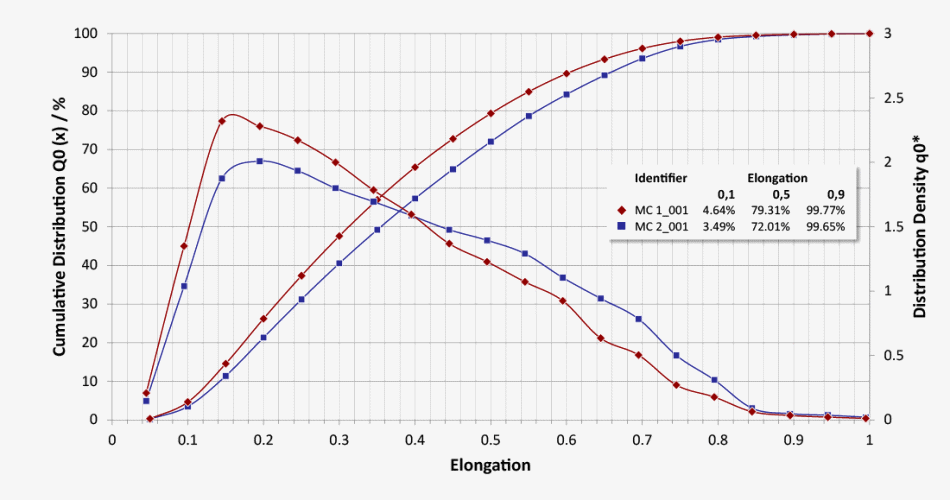
Q0 number distribution of elongation of the fibers of two methylcellulose batches (ratio of diameter to length of a fiber)
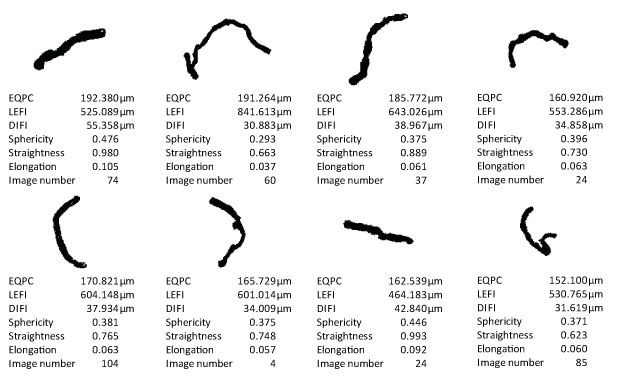
Illustrative representation of individual methyl cellulose fibers in the particle gallery
A pharmaceutical instrument design with outstanding availability, long service life and quick and easy-to-clean measuring sections ensures the high utilization and recoverability of systems. Highly skilled service staff at Sympatec assist in the preventive system maintenance, recertification and qualification. Innovative development laboratories and manufacturers or pharmaceutical excipients also rely on expertise from Sympatec which is a specialist for sophisticated formulations and a global pharmaceutical manufacturer.
- Good comparability of results at different locations
- Quick and reproducible measurement of particle size and particle shape
- Easy handling and cleaning of the measurement system
- Reliable and complete dry dispersion
- Display of suitable shape parameters for quality assessment
- No sample carryover
- Process-oriented control
Better Particles with Best Instruments
The QICPIC image analysis system from Sympatec ensures reliably precise and quick control of both the shape and fiber characteristics and the particle size of all powdered excipients. These systems are used in the laboratory for the development of pharmaceutical forms and in the process for quality control in volume production.
Reproducible dispersion of larger sample quantities for statistically reliable measurement results is ensured by RODOS dry dispersion. Even at remote production sites, Sympatec’s stable dry measuring method provides unmatched comparability of measurement results from system to system, as well as the best repeatability of individual measurements.
Measurement methods developed for a range of product qualities can be transferred to other systems as a standard operating procedure (SOP). Confidence in the premium quality of the products is justified by the consistently high product qualities and batch uniformities which can be precisely controlled.
Fast and Reliable In-Process Quality Control
The powerful components of the QICPIC system are also available for control and monitoring in the manufacturing process. The PICTOS family has been especially developed for dry and wet on-line applications and integrates dispersion and sensor technologies in a robust, GMP-compliant design. An integration into control systems for processes or for automated laboratories is realised with common communication standards such as Modbus®, Profibus® or OPC (Open Platform Communications) with TCP/IP, RS232 or RS485. The system performs the measurement and reports back the measurement results to the control system.

This information has been sourced, reviewed and adapted from materials provided by Sympatec GmbH.
For more information on this source, please visit Sympatec GmbH.