Aeroallergens like dust mites, pollens, dogs' and cats' dander and mold can influence the respiratory system and these allergens can be the consequences of respiratory diseases such as asthma and allergic rhinitis(1).
The identification and chemical characterization of these allergens will enable the development of the Raman spectra database, to more certainly and quickly establish their presence in indoor and outdoor air and also to quantify them.
Historically, the identification of aeroallergens has been performed via UV-visible and Infra-Red, fluorescence spectroscopies(3,4,5,6,7) but also by immunobiological techniques like immunoblotting or ELISA(8,9).
These biological identifications were meant to establish what type of proteins in the allergen particles were responsible for the allergy.
So, in this study, morphological identification of different types of pollen grains from commercial samples, fresh flowers, animals' dander, and dust mites will finally grant their chemical characterization by Raman spectroscopy, using optical microscopy and particle size analysis, which supplies information on the diameter of particles.
This spectroscopy method should provide information about their chemical composition in proteins, lipids, and nucleic acids.
Instruments and Techniques
Raman Spectroscopy is a non-destructive chemical analysis method which supplies detailed information about phase and polymorph, chemical structure, crystallinity and molecular interactions. It is based upon the interaction of light with the chemical bonds within a material.
Figure 1. Experimental systems used for these experiments. Left: LA-960 Particle Size Analyzer. Right: LabRAM Soleil Raman micro-spectrometer.
Raman is a light scattering method, whereby a molecule scatters incident light from a high intensity laser light source. Most of the scattered light does not provide useful information, as it is at the same wavelength (or color) as the laser source – this is known as Rayleigh Scatter.
Yet, a small amount of light (usually around 0.0000001%) is scattered at different wavelengths (or colors), which depend on the chemical structure of the analyte – this is known as Raman Scatter.
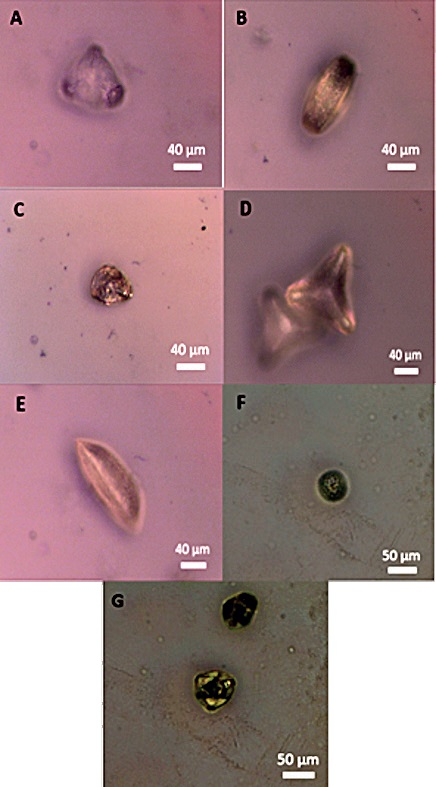
Figure 2. Optical microscopy images of pollen grains from fresh flowers. A: Camellia pollen, B: Japanese Cherry tree pollen, C: Orange Rose pollen, D: Flower thistle pollen, E: Orange Lily pollen, F: Grass pollen, G: Oak pollen.
The system which is utilized is a HORIBA LabRAM Soleil. It incorporates powerful and unique functions in a high performance, reliable system. This system is ideally suited to the research and analytical lab. It is fully confocal, not compromising image quality, depth or spatial resolution.
Furthermore, the ParticleFinder option in the LabSpec 6 software of the spectrometer, enables the user to identify the particles that make up an unknown sample. Particle Size analysis is based on laser diffraction, the main idea in laser diffraction is that a particle will scatter light at an angle established by the size of the particle.
Smaller particles scatter light at wide angles, whilst larger particles scatter light at small angles. A collection of particles generates a pattern of scattered light defined by intensity and angle which can be transformed into a particle size distribution result.
A HORIBA LA-960 is the system employed for this work. It combines the most popular modern sizing method with state-of-the art refinements to measure wet and dry samples measuring from 10 nanometers to 5 millimeters.
Morphological Identification
1. Pollen Grains
a) Identification by Optical Microscopy
An initial observation of the pollen grains collected on fresh flowers and from commercial samples was possible due to the Raman spectrometer microscope.
An observation with a 50x long working distance objective is enough as the grains were quite large. In this instance, the differentiation between the pollen grains is made by appearance.
b) Identification by Particle Size Analysis
To ensure a mixture of grains, this analysis was performed on pollen grains of orange lily flowers but also from balls of pollen grains of different colors. This choice of samples, particularly the mixture, is used to confirm or invalidate the interest of this method to the differentiation of the pollen grains between them.
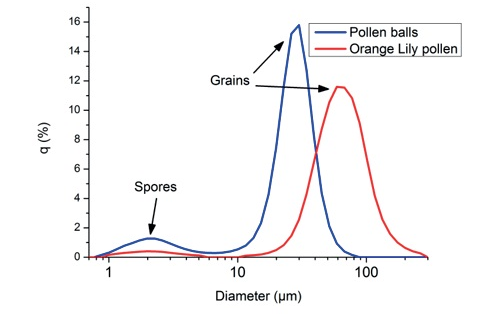
Figure 3. Particle size distributions of different pollen grains.
It would be more effective than a microscopic observation, as this method is quicker. The separation of pollen grains here is by size and not by appearance.
Regarding the distribution of the different pollen grains, the Lily pollen has an average diameter of 63 µm, the spores have an average diameter of 2 µm, and the mixture of pollen balls have an average diameter of 24 µm.
The morphological identification of pollen grains by particle size analysis supplied information on the diameter of these particles, but this information is not sufficient in the mixture sample as it does not enable the user to clearly identify each type of pollen grain.
As particle size measurements are unable to supply chemical information, structural identification of the pollen grain was not possible and the utilization of a molecular spectroscopic analysis like Raman spectroscopy is vital to provide additional information to the previous assumption.
2. Animals' Dander and Dust Mites
Due to their large size (Figure 4), these aeroallergens were observed using the 50x long working distance objective of the microscope which was sufficient. Optical microscopy enables the user to identify each pollen grain and to differentiate them from each other but also from the other allergens.
However, as their topography is not homogenous, it is more complicated to identify dander and dust mites. In addition, for an inexperienced person, it will be challenging to attribute a pollen grain to its species.
In this way, Raman spectroscopy coupled with microscopy will be a key method to characterize these allergens and to identify them in an unknown mixture.
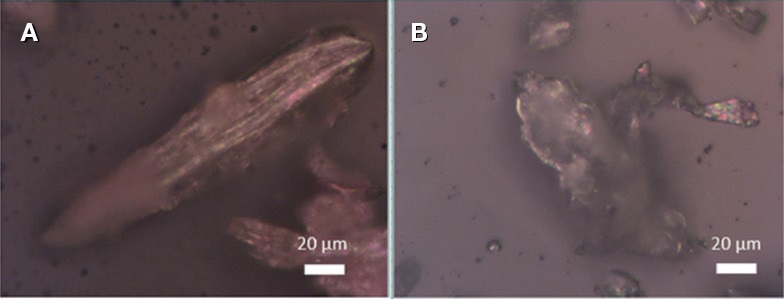
Figure 4. Optical microscopy images of commercial samples. A: Dander, B: Dust mite.
Chemical Characterization by Raman Spectroscopy
Initially, aeroallergens were spectrally characterized individually, resulting in Figure 5. These Raman spectra are reference spectra, they were measured on single particles of allergens.
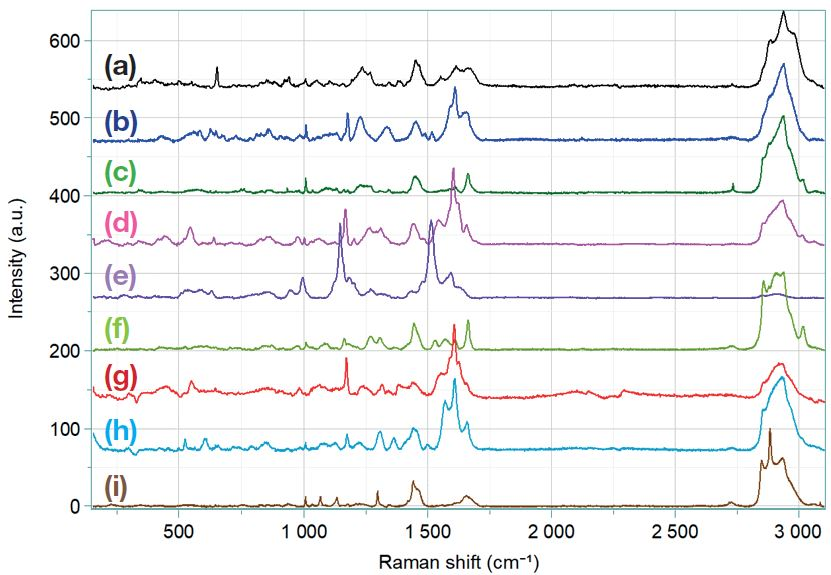
Figure 5. Reference spectra of aeroallergens. (a): Dander, (b): Cherry tree pollen, (c): Camellia pollen, (d): Lily pollen, (e): Rose pollen, (f): flower of Thistle pollen, (g): Oak pollen, (h): Grass pollen, (i): Dust mite.
Pollen grains have a similar spectrum, some bands are different by their Raman shift and intensity as the one at 1.170 cm-1, 1.600 cm-1 and 1.650 cm-1. All the bands present on the spectra exhibit the presence of lipids, proteins, sugars and nucleic acids(2,4).
The dander and dust mites’ spectra follow the same pattern as the pollen grains’ spectrum. Yet, the bands are very different, these differences between the Raman spectra will facilitate the determination of the allergens in a mixture sample.
So, ParticleFinder module LabSpec6 Software Suite allows the user to measure one spectrum by particle, locating the centers of the particles which are present in the microscopic image.
Next, the identification of each particle in the unknown mixture is possible by cross-checking the reference spectra gathered with the clustering separation, even if there is an important number of it. Figure 6 shows an example of such analysis.
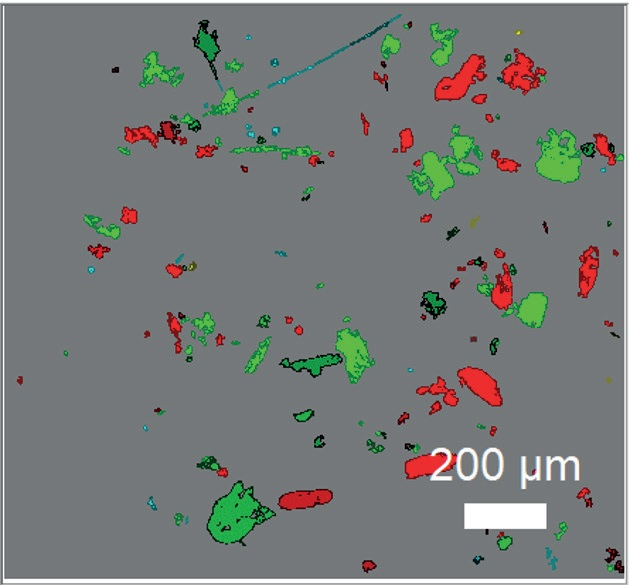
Figure 6. ParticleFinder analysis of 174 particles of aeroallergens. Red: Dust Mite, Green: Dander, Light blue: Grass pollen, Yellow: Lily pollen.
So, this variety of analysis enables the characterization of a mixture of aeroallergens not only in terms of the size of the particles, but also of chemical composition. As this one is based on the spectral fingerprint of the individual particles, is also has a high reliability on the identification.
Conclusion
In this article, the purpose was to identify aeroallergens in an unknown sample. As this one is based on the spectral fingerprint of the individual particles due to optical microscopy, which enables the user to morphologically identify them and then to measure their Raman spectra in a way of chemical characterization.
Considering reference spectra of these particles, they could finally be found in the mixture sample. So, Raman microscopy is a promising method for the identification in real time of aeroallergens like dust mites, pollen, grains, and animal’s dander in the outdoor and indoor air.
So, by taking an air sample and then filtering it, it is possible to analyze and especially quickly determine what type of aeroallergens are present inside. This could enhance the health awareness by preventing the risks of allergies for people who may be affected by it.
References and Further Reading
- https://www.inserm.fr/information-en-sante/ dossiers-information/allergies
- A. Guedes, H. Ribeiro, I. Abreu et al., Talanta 2014; 119, 473-478.
- B.G. Pummer, H. Bauer, H. Grothe et al., J. Raman Spectrosc. 2013; 44, 1654-1658.
- F. Schulte, J. Lingott, U. Panne & J. Kneipp, Analytical Chemistry 2008; 80 (24), 9551-9556.
- B. Zimmermann, Applied Spectroscopy 2010; 64(12), 1364–1373.
- M; Bağcıoğlu, B. Zimmermann, A. Kohler, PLoS ONE 2015; 10(9): e0137899.
- B. Zimmermann, Z. Tkalčec, A. Mešić & A. Kohler, PLoS ONE 2015; 10(4): e0124240.
- S Spitzauer, C. Schweiger, H. Rumpold et al., Int Arch Allergy Immunol 1993; 100:60-67.
- Y. Cui, Q. Wang & H. Jia, Transl Allergy 2018; 8: 14.
Acknowledgments
Produced from materials originally authored by Nihal Nagib, Thibault Brulé and Nicolas Buton from HORIBA FRANCE SAS.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.