Polyesters are polymers composed of two compounds; a dicarboxylic acid and a diol. Polyethylene terephthalate (PET) tends to be the most common polyester on the market. Fundamentally, when carrying out PET Synthesis, the primary task involves combining terephthalic acid or dimethyl terephthalic acid with ethylene glycol and heat.
Continuous process monitoring of both the component and product streams can often improve the production of polyesters as this enables the utilization of all of the information collected in real-time. Near-infrared analyzers are a tool that can assist process engineers and technicians to make well-informed decisions for process optimization.
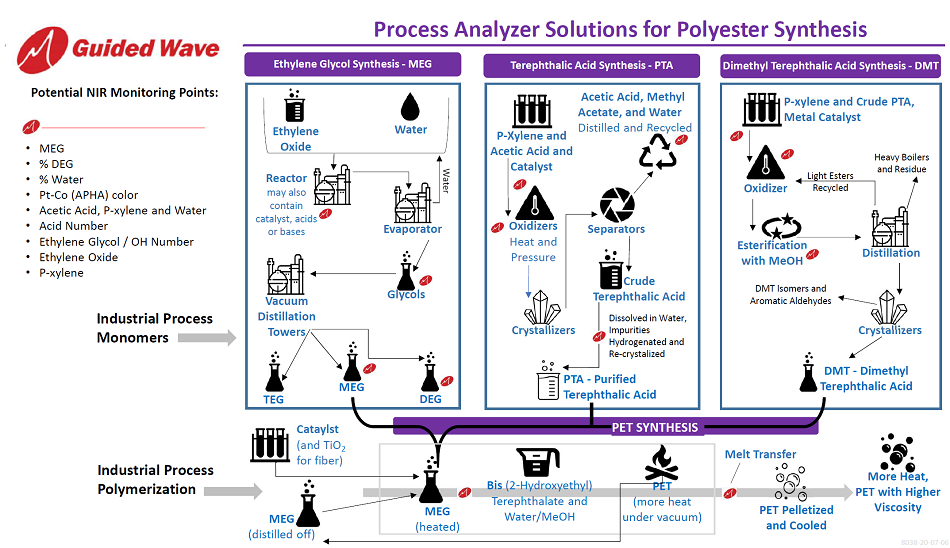
How Near-Infrared Analyzers are Beneficial to Ethylene Glycol Synthesis
Typically, the PET synthesis process starts by mixing ethylene oxide and water in a thermal hydration reactor at about 200 °C. Reaction kinetics such as the concentration of excess water as well as the ratio of glycol reaction products are easily monitored using near-infrared analyzers.
The ethylene oxide to ethylene glycol thermal conversion process tends to yield about 90–92% monoethylene glycol (MEG) and 8–10% bulkier glycol products, mainly diethylene glycol (DEG) and triethylene glycol (TEG). Near-infrared measurements can be carried out on the various glycols as well as in the ethylene carbonate to ethylene glycol reactors.
The outcome is a water-glycol mixture coming from the thermal hydration reactor which is then delivered to multiple evaporators where any excess water can be recovered and recycled. Subsequentially, vacuum distillation enables the division of the water-free glycol mixture; finally separating it into MEG and the higher glycols. Near-infrared analyzers can be utilized at this point to verify yield and other useful parameters.
Most MEG is reacted with para-terephthalic acid (PTA) to generate polyester fibers for use in textiles and garments and polyethylene terephthalate (PET) plastics most commonly found in food and drink packaging. Moreover, to prevent an undesirable and off-color product the APHA color can also be monitored to help avoid a discolored (yellow) monomer.
How Near-Infrared Analyzers are Beneficial to Terephthalic Acid and Dimethyl Terephthalic Acid Synthesis
Synthesis of Terephthalic Acid and Dimethyl Terephthalic Acid can be achieved by reacting to P-xylene with cobalt–manganese–bromide catalyst, acetic acid, and air. The Process Insights Clearview DB analyzer facilitates real-time quantitative monitoring of this caustic reaction. A Kalrez sealed or gold brazed single-sided insertion probe (SST) can then be directly installed into the reaction vessel for continuous process monitoring and optimization. Additionally, in a high-risk or corrosive environment, the sample probe can be constructed using Hastelloy C276.
How Near-Infrared Analyzers are Beneficial to PET Synthesis
Throughout batch polymerization, dimethyl terephthalate (DMT) reacts with ethylene glycol in esterification to produce monomer alcohol, which then polymerizes with terephthalic acid (TPA). By way of contrast, the continuous method involves a polymerization between TPA and ethylene glycol, so DMT is not required. Each of these methods requires heating either the DMT or TPA with ethylene glycol to about 536 ºF for 30 minutes at atmospheric pressure, following this the mixture is placed under vacuum for 10 hours.
Near-Infrared analyzers including the Clearview DB and NIR-O Process analyzers can be employed to offer in-situ monitoring of the cross-linking under vacuum conditions. During melt transfer, out of specification products can be identified with a high-temperature shuttle probe and Near-Infrared monitoring.
Therefore, by continually monitoring the molten polymer stream for Acid Number, costs can be reduced by preventing any off-spec product from reaching the extrusion or pelletizer. Petrochemical reactions, including polyester (PET) synthesis, profoundly benefit from constant monitoring using near-infrared analyzers. Thus, incorporating Near-Infrared analyzers into synthesis operations can optimize processes and significantly reduce any operating costs.

This information has been sourced, reviewed and adapted from materials provided by Process Insights – Optical Absorption Spectroscopy.
For more information on this source, please visit Process Insights – Optical Absorption Spectroscopy.