The quality and value of a product are inherently connected. In the case of oils and fats, this may be complex to establish by looks alone. Particularly in high-cost natural oils from plant sources, the rancidity of these products is a factor that can instantly decrease the sale price.
Oils that maintain stability over extended periods of time have a higher value as they produce an end product of higher quality, for example when used in cosmetics or food products.
Rancidity arises naturally over time as oils and fats oxidize and age. Certain oils already include antioxidants that extend their lifespan, depending on the source. Other oils may be more vulnerable to aging and further antioxidants are required to prolong usability and shelf life.
Identifying rancidity can be achieved using several techniques, such as measuring the peroxide value or acid number. These tests only provide details about the product’s current state, where no information is given about the remaining shelf life. The Rancimat method is an analytical technique that can quantify this time span until spoilage.
In this method, the sample is put through an accelerated aging process and the induction time establishes how long the oil will be viable. This technique allows manufacturers to guarantee and validate the quality and value of their products to customers in the cosmetics and food industries.
What are Natural Oils?
All oils acquired naturally from plants are known as natural oils. This categorization does not say anything about the handling of the oil—whether it was acquired natively, by extraction, or by other techniques. Oils are grouped differently according to how they are processed:
- Refined oils are normally hot-pressed at 100 °C and then refined according to the oil. For refined oils, the oil is filtered and hardened again, and preservatives may be included to extend shelf life.
- Unrefined oils are normally cold-pressed but can be heated up to 60 °C in the pressing process.
- Oils that are not subjected to any external heat input during pressing are known as cold-pressed oils. The only heat in this process is produced by pressure and friction.
- Virgin oils are cold-pressed, particularly in the case of olive oil, where the quality grade of a native, extra virgin is given based on acidity. The acid value (SC) should be less than 0.8 g/100 g to label olive oil for sale as extra virgin.
In the cosmetics market, a range of natural oils is utilized which are not significantly different from food oils.
Composition of Oils
Triglycerides are the main component of oils (officially called triacylglycerols, as stated by IUPAC). Natural oils mainly consist of triglycerides, where one glycerin molecule is esterified with three fatty acids (Figure 1).
These fatty acids can have various chain lengths, and their composition is strongly related to the raw material from which they are sourced.
Unsaturated fatty acids are the term for fatty acids which include double bonds. A fatty acid-containing more double bonds can be oxidized easier. Essential fatty acid esters is another term for these unsaturated glycerides.
Natural oils comprise various combinations of these triglycerides. Bound fatty acids are what fatty acids are called when they are bound to the glycerin molecule. In time, these fatty acids disintegrate. Free fatty acids are acquired and the esters are split. These free fatty acids are quantified by the acid number.
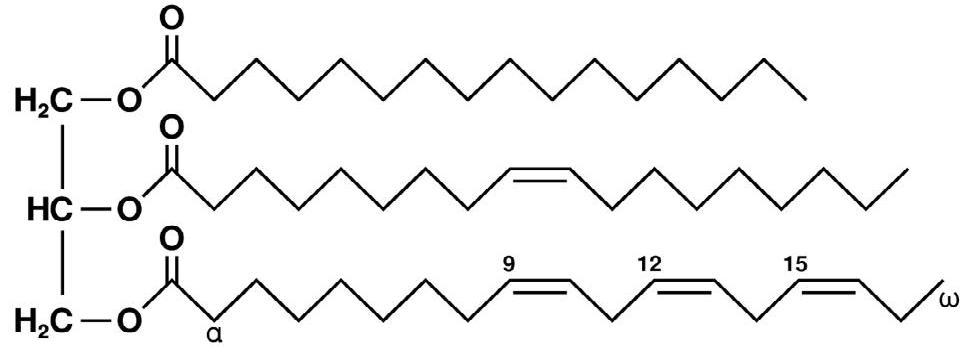
Figure 1. Example of an unsaturated triglyceride (C55H98O6): (L) glycerol, (R) from top to bottom: palmitic acid, oleic acid, and alpha-linolenic acid. Image Credit: Metrohm AG
Oleic acids, with a double bond at the ninth C-atom, are the most widespread fatty acid esters present in natural oils. Compared to polyunsaturated fatty acids, they are relatively resistant to oxidation. Linoleic acid is assigned to the category of omega-6 fatty acids and contains two double bonds. It can be oxidized with ease.
Linolenic acid contains three double bonds and is an omega-3 fatty acid. Compared to linoleic acid, it is even easier to oxidize. Omega-6 and omega-3 fatty acids are both essential fatty acids, which means they must be consumed with food as the body cannot create them on their own.
The oils acquired vary in composition and in their characteristics as cosmetic oils or food oils according to the type of plant and seed from which they are found.
All vegetable oils are primarily composed of stearic acid, palmitic acid, linoleic acid, linolenic acid, and oleic acid. Along with these fatty acids, the oils also include a range of antioxidants like vitamin E and vitamin A.
Rancidity – How Does it Arise?
Freshly pressed oils include unsaturated fatty acids bound to glycerin with various numbers of double bonds and chain lengths. The oxygen found in the air oxidizes these. This process of oxidation usually develops very slowly at room temperature.
All-natural oils experience aging over time. When the fatty acids oxidize, rancidity is produced. This autooxidation of fats and oils is a radical reaction and occurs in three stages.
The initiation stage is the first to occur, which is where lipid radicals are produced from triacylglycerols. Oils normally include traces of hydroperoxides, which may have been generated by lipoxygenase processes in the plant prior to and during the extraction of oil.
Usually, the major initiation pathway in fats and oils is the initiation by homolytic cleavage of hydroperoxides, which is a comparatively low energy reaction. This reaction is normally catalyzed by metal ions (such as iron), which means it is important to ensure there are no metal ions present in the product.
Propagation reactions (chain propagation) arise after initiation, where one fatty acid radical is changed into different fatty acid radicals. A hydrogen atom is normally removed during this reaction, or an oxygen radical has a reaction with the fatty acid. Compared to the initiation reactions, the reaction enthalpy is comparatively low so that chain propagation is quicker in relation to the initiation reactions.
As the adjacent double bonds reduce the bond dissociation energy of the C-H bond, hydrogen abstraction arises more efficiently at the methylene group located between two alkene groups in a polyunsaturated fatty acid.
Alkoxy radicals produced by the decomposition of hydroperoxides may disintegrate and emit volatile aldehydes, hydrocarbons, or alcohols that are no longer bound to the glycerol backbone.
Volatile aldehydes are especially critical as they contribute to the aroma of oxidized oils. One of these aldehydes, hexanal, is usually investigated to evaluate the creation of secondary oxidation products during the oxidation of lipids.
Effect of Antioxidants
Antioxidants are the term for substances that are able to prevent or delay the growth of rancidity or other deterioration of material as a result of oxidation. Commonly, antioxidants are known to hinder the development of flavor changes by prolonging the induction stage of oxidation.
Adding antioxidants after this stage is normally ineffective as substances with a low molecular weight are already present.
Antioxidants can delay or prevent oxidation in two ways. Compounds are called a primary antioxidant when they scavenge free radicals, or they are known as secondary antioxidants when they use a process that does not include the direct scavenging of free radicals.
Phenolic compounds like vitamin E (α-tocopherol) are included in primary antioxidants. During the induction stage, these components are consumed. Secondary antioxidants function through a range of mechanisms, such as the uptake of oxygen, the conversion of hydroperoxides to non-radical species, or the complexation of metal ions.
Secondary antioxidants usually will only display antioxidant activity when a second component is included. This can be noted in compounds like citric acid, which are only effective in the presence of metal ions, and reducing agents like ascorbic acid, which are effective in the presence of other primary antioxidants or tocopherols.
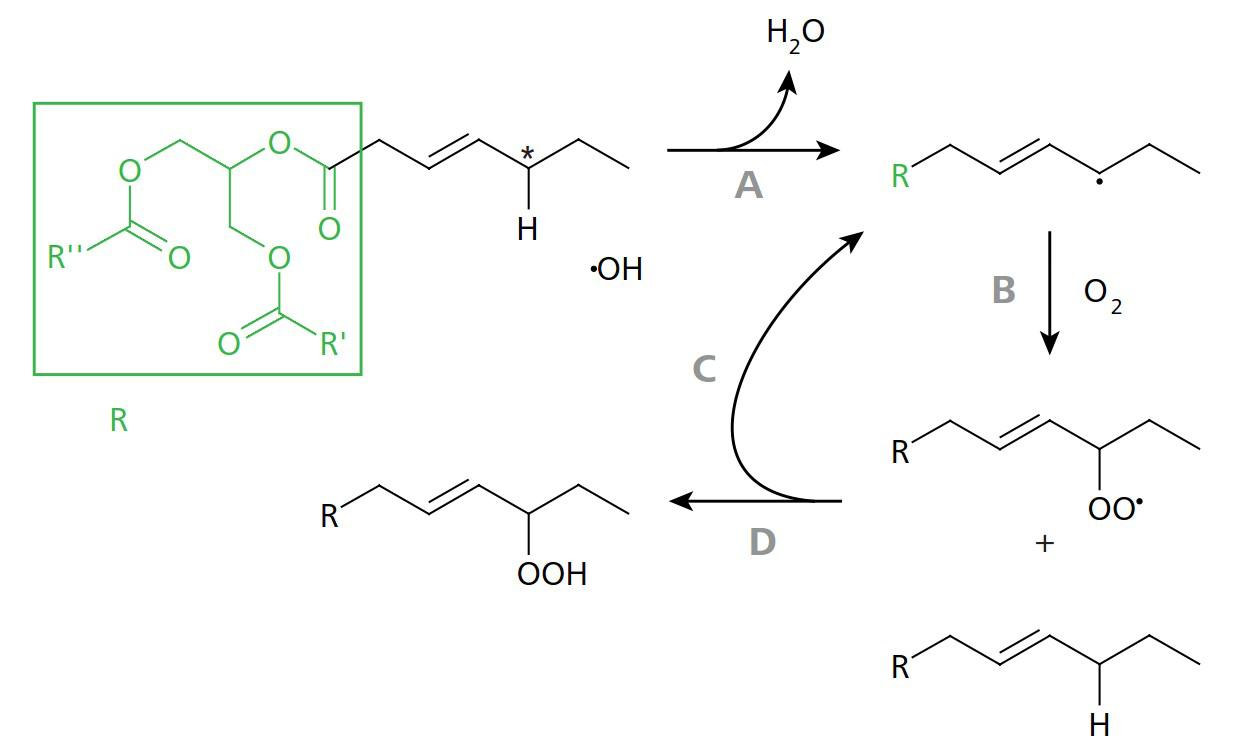
Figure 2. Scheme of peroxidation of a monounsaturated fatty acid. A: The methylene group (marked with an asterisk) is particularly susceptible to the cleavage of an H atom due to its position adjacent to the double bond. This leads to a reaction with the hydroxyl radical. B: The reactive radical binds to molecular oxygen from the ambient air. C, D: A reaction with a "fresh" fatty acid produces a peroxide on the one hand and a free radical on the other – a chain reaction begins. Image Credit: Metrohm AG
Natural antioxidants (such as tea extracts and rosemary extract) are additionally found on the market along with synthetic antioxidants. The oxidation stability of the natural oils can be extended if required when these are added to the natural oils.
A typical effect of this type of antioxidant is that they generate a delay time, known as the induction period IP. This lasts until around 90% of the antioxidant is destroyed and normally corresponds in duration (or length) to their concentration. The oxidation of fatty acids occurs very slowly during this delay time, but oxidation efficiently accelerates once this is used up.
Determination of the Oxidation Stability with the Rancimat
An accelerated oxidation test is a technique to determine oxidation stability. In this investigation, a sample is held at a fixed temperature in a closed reaction vessel, and a consistent flow of air is passed through the sample. The oxidation of fatty acids in the sample causes peroxides to be formed as primary oxidation products.
The fatty acids are entirely destroyed after some time, and low-molecular-weight organic acids (particularly acetic and formic acid) are produced as secondary oxidation products, along with additional volatile organic compounds.
The airflow conducts these secondary oxidation products into a measuring vessel which includes distilled water as an absorption solution. The conductivity of this water is constantly monitored.
The increase in conductivity in the measuring vessel signifies the formation of volatile carboxylic acids in the sample. The time it takes for the secondary oxidation products to form is known as the induction time and indicates the sample’s oxidation stability.
Two distinct measuring blocks are included in the 892 Rancimat which provides the simultaneous measurement of up to eight samples. Each sample measurement can be started independently.
A measuring station is therefore instantly available for a new sample at the end of the measurement, and the instrument can be utilized to its complete capacity. The clear and simple construction of the measuring instrument also enables the user to monitor a larger quantity of samples.
A built-in pump generates the airflow through the sample which can be automatically managed in relation to the method settings. It is not required to use gas pressure lines for the air supply.
On the Metrohm 892 Professional Rancimat, measurement preparations are very straightforward. The sample is weighed into the reaction vessel and distilled water is used to fill the measuring cell. The measurement can begin after two tubes are connected.
Cost-effective disposable reaction vessels are used in the 892 Professional Rancimat. On the one hand, this removes the often time-consuming cleaning process at the end of the determination, and on the other hand, it enhances the reproducibility of the measurements and the accuracy of the results.
The conductivity measuring cell is built in the lid of the measuring vessel. The cell is submerged in the distilled water when the lid is put on.
Simultaneously, electrical contact is established with the measuring electronics in the instrument, removing the requirement for complicated wiring of the measuring input. The conductivity electrode is additionally impressive with its robust construction. It is simple to thoroughly clean it with a brush.
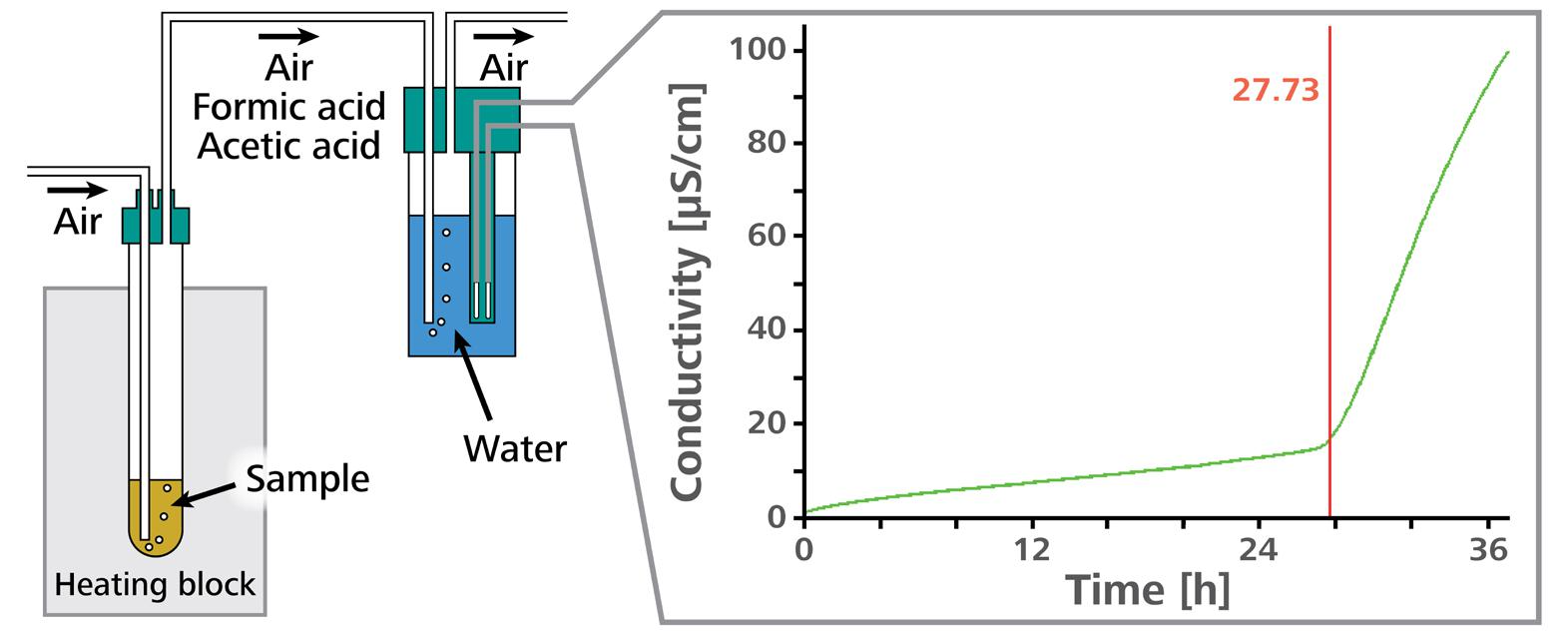
Figure 3. Schematic representation of a measurement with the Rancimat. Image Credit: Metrohm AG
Figure 4. By using inexpensive disposable reaction vessels, the 892 Rancimat can be used efficiently, as time-consuming cleaning steps are no longer necessary. Image Credit: Metrohm AG
The Rancimat can achieve results within mere hours, compared to what can take weeks, months, or even years on the shelf.
There is a causal relationship between the composition of the oil and the induction time. A higher acid number is the result of more unsaturated fatty acids being present, and the induction time is shorter when there are fewer antioxidants (vitamin A, vitamin E) found in the oil. A different induction time is achieved according to the natural oil age of the sample and its composition.
Oxidation Stability of Cosmetics
A high quantity of vegetable fats and oils are found in a number of natural cosmetics, and also in different high-quality cosmetic products. As an example, cocoa butter is an ingredient found in several body and lip care products.
These cosmetics spoil over time, unlike paraffin-based products. This is caused by the oxidation of the vegetable fats included in the product, among other reasons. According to the fat content, the samples can either be directly quantified with the 892 Professional Rancimat, or the isolated fat can be measured after cold extraction with petroleum ether. When natural oils are used, these can be directly investigated.
The induction times for a range of natural oils are displayed in the following table. The results can be employed to identify what each respective oil provides in terms of results with the respective technique.
Source: Metrohm AG
| Natural oil |
Temp.
(°C) |
Induction
period (h) |
| Acai fruit oil, org. |
120 |
16.46 |
| Algae oil |
120 |
2.63 |
| Almond oil cold-pressed, org. |
120 |
3.10 |
| Almond oil cold-pressed, org. and demeter |
120 |
2.64 |
| Apricot kernel oil cold-pressed,org. |
120 |
7.51 |
| Argan oil cold-pressed, org. |
120 |
7.14 |
| Argan oil deodorized, org. |
120 |
5.56 |
| Argan oil, org. |
120 |
6.52 |
| Avocado oil |
120 |
2.34 |
| Avocado oil cold-pressed, org. |
120 |
8.39 |
| Avocado oil, org. |
120 |
4.70 |
| Baobab, org. |
120 |
3.34 |
| Beeswax |
160 |
1.87 |
| Black seed oil, org. |
120 |
1.58 |
| Cashew oil CO2 extraction, org. |
120 |
6.55 |
| Castor oil |
150 |
10.02 |
| Chia oil cold-pressed, org. |
120 |
3.49 |
| Coconut oil, org. |
160 |
76.05 |
| Coconut oil, org. |
120 |
1.70 |
| Cranberry seed oil cold-pressed, org. |
120 |
0.64 |
| Evening primrose oil |
120 |
0.72 |
| Evening primrose oil, org. |
120 |
0.97 |
| Hemp oil, org. |
120 |
1.23 |
| Hemp seed oil cold-pressed, org. |
120 |
2.90 |
| Hexadecan-1-ol, cetyl alcohol |
140 |
25.01 |
| Jojoba oil cold-pressed, org. |
120 |
24.33 |
| Jojoba oil cold-pressed, org. and demeter |
120 |
25.01 |
| Jojoba oil refined, org. |
120 |
16.26 |
| Jojoba oil, org. |
120 |
23.73 |
| Jojoba oil, org. |
140 |
4.18 |
| Lanolin, wool wax |
120 |
3.11 |
| Linseed oil, org. |
120 |
0.80 |
| Macadamia nut oil, org. |
120 |
10.99 |
| Mango butter, refined |
120 |
11.15 |
| Mango butter, refined |
130 |
5,14 |
| Marula oil cold-pressed, org. |
120 |
44.11 |
| Neem oil, org. |
110 |
5.74 |
| Octadecan-1-ol, stearyl alcohol |
140 |
3.47 |
| Pomegranate oil, org. |
100 |
0.86 |
| Sacha inchi oil cold-pressed, org. |
120 |
1.11 |
| Sea buckthorn oil, org. |
120 |
35.58 |
| Sea buckthorn seed oil CO2 extraction, org. (n = 3) |
120 |
0.39 |
| Sesame oil |
120 |
3.75 |
| Sesame oil, org. |
120 |
3.65 |
| Shea butter raw, org. |
120 |
0.91 |
| Shea butter refined, org. |
120 |
3.91 |
| Shea butter, org. |
120 |
9.18 |
| Shea butter, refined |
120 |
8.39 |
| Shea butter, unrefined |
120 |
2.85 |
| Shea nut oil, refined |
120 |
1.51 |
| Sweet almond oil, org. |
120 |
1.47 |
| Wheat germ oil CO2 extraction, org. |
120 |
1.73 |
Conclusion
Traditional fat measurements like the peroxide value or acid number only identify the current status of fats and oils. They can only be utilized to make restricted predictions about the product’s remaining shelf life, which is a crucial parameter when establishing the value of oils created for cosmetic or food applications.
This knowledge gap can be solved by using the induction time as a further fat index parameter, which is easily quantified with the Rancimat from Metrohm.
The induction time indicates the number of double bonds, existing antioxidants, and existing pro-oxidants, and the interaction of bound and free fatty acids at high temperatures. This provides a complete picture of the quality of an oil and how it will function over time.
The extraction technique employed plays a crucial role, especially in situations where natural oils include a high amount of unsaturated fatty acids. In this example, the Rancimat technique delivers a clear basis for choosing which technique is most appropriate.
Rancidity in a natural oil cannot be reversed. This process can be delayed or even prevented through the addition of natural antioxidants (for example, vitamin E, tea extract, or rosemary extract) in advance if it is known that the natural oil will become rancid in the future.
Quantifying the induction time using the Rancimat enables manufacturers to get as much value from the oil as possible by establishing the shelf life and deciding if additional actions are required to prolong its life prior to sale.
References and Further Reading
- ISO/TR 18811:2018 Cosmetics – Guidelines on the stability testing of cosmetic products https://www.iso.org/standard/63465.html
- ISO 16128-1:2016 Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients and products — Part 1: Definitions for ingredients https://www.iso.org/standard/62503.html
- ISO 16128-2:2017 Cosmetics — Guidelines on technical definitions and criteria for natural and organic cosmetic ingredients — Part 2: Criteria for ingredients and products https://www.iso.org/standard/65197.html
- Stability Application Note R-029: Oxidation stability of cosmetic raw materials https://www.metrohm.com/en_in.html
- Oxidation in foods and beverages and antioxidant applications Volume 2: Management in different industry sectors ISBN 978-0-08-101457-8

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.