Understanding the Air Cooled Reflux System
Increased demand and emphasis on safety and sustainability in the chemical laboratory have activated the development of new reflux condensers without water cooling. With the Findenser™, Heidolph is able to provide an efficient, air cooled reflux system.
What are the pros and cons of reflux condensers? What are the general aspects to be taken into consideration when buying one? The Heidolph 'Reflux Condenser Buyer’s Guide' provides the answers to the key questions. For all other matters, the Heidolph Customer Support is on hand to offer advice.

Image Credit: Heidolph North America
Why Refluxing?
Refluxing is one of the most frequently used working methods for organic synthesis. Most chemical reactions are accelerated by an increased reaction temperature or only performed under heat conditions.
The use of reflux condensers prevents evaporation of the solvent of a reaction mixture even when it is heated for long periods of time.
Air Cooled or Water Cooled: What is the Difference?
As simple as it is ingenious, the refluxing principle sees the boiling vapor from the solution in the flask rising vertically into the glass column. There, it condenses on the cooling surfaces and descends into the flask as the liquid condensates.
Cooling inside a reflux occurs in-line with the heat exchanger principle. The following is true for the cooling of surfaces: the larger the surface, the better the behavior (of cooling).
Water cooled: In a majority of the cases, the coolant used is tap water. Flowing around the reflux column like a water sheath it coils on the exterior of the column of which the boiling vapor rises. Convexities on the inner walls of the column or cooling coils expand the cooling surface. (Fig. 1 Functional principle of a water-cooled reflux condenser)

Figure 1. Water cooled reflux system. Image Credit: Heidolph North America
Air cooled: Reflux condensers with air cooling are available in a wide variety of systems. In low-priced systems, heat exchange with the ambient air only occurs on the outer surfaces of the glass column. Thus, the column must be set suitably high enough to dissipate heat to the environment.
The design of the reflux condenser system Findenser™ is much more complex and fitted with a finned aluminum jacket, which multiplies the heat exchanger surface in a way that is still compact.
Safety in the Laboratory has Many Facets
The speed at which a hose can come off a water cooled reflux condenser and the extent of the damage caused by flooding in a laboratory building then an air cooled laboratory condenser can be considered a true alternative.
In the worst-case scenario, laboratory equipment worth thousands of dollars can be damaged beyond repair overnight. After all, around 2.5 liters of water per minute flow through a water cooled reflux system.
The lack of any tubing – as in air cooled reflux condensers – means that there the risk of tubing coming into contact with hotplates is eliminated and furthermore requires just relatively a simple setup with less space required in the fume hood.
Water Consumption and Cooling Costs
Extrapolated, an average of 150 liters of water per hour flows through a water-cooled reflux condenser (Fig. 2 Diagram Water consumption in comparison).
Fortunately, most laboratories have installed recirculating chillers or central water recirculating systems to limit the vast amounts of water consumption during refluxing. However, cooling the coolant can result in a negative impact on the energy balance.
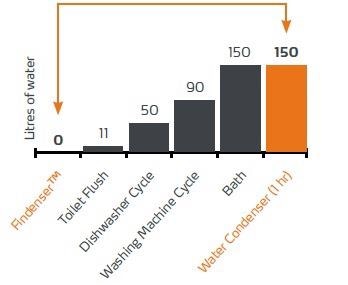
Figure 2. Diagram: Average water consumption of common household appliances compared to the Findenser™ and the water cooled reflux condenser. Image Credit: Heidolph North America
An as Wide as Possible Range of Applications
Conventional, air cooled reflux condensers have a considerably narrower range of applications in contrast to water cooled devices. This is due to the very nature of physics because a low cooling capacity means they are not suitable for highly volatile, low boiling solvents - this applies to the most common solvents used for syntheses.
Attention should be paid to the application range when selecting a reflux condenser. While the Findenser™ is also air cooled, it provides exceptional cooling capacities due to its highly thermal conductive aluminum cooling fins and making it appropriate for around 95% of all chemical syntheses.
These common solvents are already used successfully with the Findenser™ for chemical synthesis without any significant volume loss:
Hydrocarbons: e.g. cyclohexane, pentane, hexane and heptane.
Chlorinated solvents: e.g. dichloromethane (DCM), chloroform.
Alcohols: e.g. ethanol, isopropanol and methanol.
Aromatic hydrocarbons: e.g. benzene, toluene and xylene.
Miscellaneous: Methyl tertbutyl ether (MTBE), acetone, tetrahydrofuran (THF), ethyl acetate, acetonitrile, dimethyl furan (DMF) and many others.
The ceiling for the boiling temperature is 155°C/311°F. If any concerns arise, contact Heidolph Customer Support.
The Best is to Test: Solvent Volume
An identifying factor for solvent loss during refluxing is the volume. Standard, air cooled reflux condensers are for the most part insufficient for larger volumes. At high volatility, too much, if not all, of the solvent is quickly lost.
Despite its considerably higher cooling capacity, even the air cooled Findenser™ can reach its limits. To avoid risk, prior to the actual test, perform a test under real conditions with highly volatile solvents such as ether; always work in the fume hood when conducting these runs.
Essential: Accurate Temperature Control
Numerous tests have discovered that with accurate temperature control the Findenser™ works with particular efficiency.
The control temperature at the hotplate should be set at 10-15 °C/50-59 °F above the boiling point of the solvent in use. If the temperature is too high or if it shifts greatly, the solvent can boil over which could damage the sample.
This is why, for subsequent reflux reactions it is necessary to pay attention to a suitable reflux condenser as well as the heat source being used.
If magnetic stirrers with a digital display or with an interface for a digital contact thermometer is the preferred choice, then Heidolph Customer Support is on hand to provide more information.
Suitable for Almost all Ambient Conditions
The greater the ambient temperature, the worse the performance. In contrast to water cooled models, ambient temperature plays a significant role in air cooled reflux condensers.
For both this and safety reasons, it is recommended refluxing in conducted the fume hood. This guarantees reproducibility and ensures that room temperature is not an issue.

Safety in the lab: Findenser™ (on the right side) takes less space in the fume hood. Image Credit: Heidolph North America
Good Price/Performance Ratio
Basic reflux condensers can already be purchased for just a small cost. However, water running expenses and cooling energy costs should always be considered before acquisition. The safety aspect should not be underestimated as in the event of flooding, the resulting damage is up to a thousand times greater.

A precise temperature control of the reaction is only possible by means of use of an external temperature sensor. Image Credit: Heidolph North America

This information has been sourced, reviewed and adapted from materials provided by Heidolph North America.
For more information on this source, please visit Heidolph North America.