Surfactants are amphiphilic molecules that contain hydrophilic and hydrophobic parts. Surfactants orient themselves at the air-water interface so that the hydrophilic part is in water and the hydrophobic part in the air when they are dissolved into water.
The surfactant molecules will be regularly arranged on the liquid surface or form micelles through self-assembly behavior if the surfactant concentration is higher than the critical micelle concentration (CMC).
It is worth considering the change of surfactant concentration and ambient temperature will result in different phase behaviors of the micelles and, so, different morphologies and sizes.
There are various types of surfactant molecules, and these can be divided into non-ionic surfactants like the CmEn series, ionic surfactants such as SDS, and copolymer surfactants.
The phase behavior of a certain surfactant is established by its specific type or chemical composition. Surfactants are used in a number of fields such as biology, chemistry, and pharmaceuticals.
In this study, through dynamic light scattering (DLS) technology, an ionic surfactant micelle SDS and a non-ionic surfactant micelle Tween 20 were examined by investigating their particle sizes and the influence of temperature on their phase behaviors.
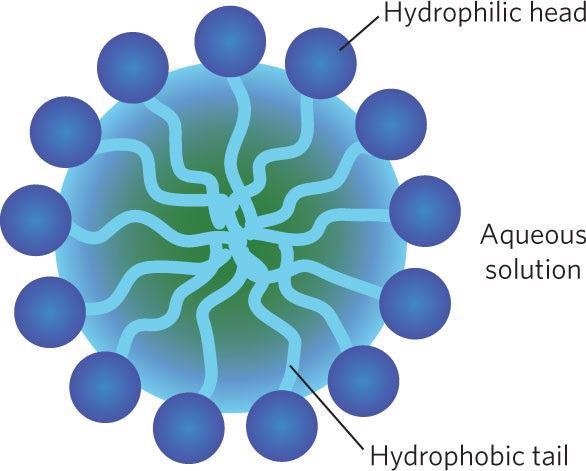
Image Credit: Bettersize Instruments Ltd.
Theory and Instrumentation
DLS measures the intensity fluctuations of the sample due to Brownian motions of particles. By using the Stokes-Einstein equation, the diffusion coefficient D is obtained and related to the particle size, i.e., the hydrodynamic diameter DH.

Where kB is the Boltzmann constant, T is the temperature, and η is the dispersant viscosity.
In this article, the surfactant samples were characterized using the BeNano 90 Zeta from Bettersize Instruments Ltd., which utilizes a He-Ne laser with the power of 10 mW and the wavelength of 633 nm.
In the BeNano 90 Zeta, single-mode optical fibers are also utilized for signal transmission to maximize the signal-noise ratio; high-speed correlators are used such that the fast-decay correlation functions for small particles can be effectively calculated.
Experiment
As shown in Table 1, two surfactant suspensions were prepared at different concentrations. The built-in temperature control system of the BeNano 90 Zeta was used to set the measurement temperature at 25℃ ± 0.1℃.
As the surfactant scattering intensity was extremely weak and the molecules were extremely small, the presence of impurities, like dust, would have a big effect on the measurement results.
For that reason, before the measurement, the samples were filtrated using a 220 nm filter. To ensure repeatability of the results, each sample was measured a minimum of three times.
Table 1. Sample Information. Source: Bettersize Instruments Ltd.
| Sample |
Concentration |
Dispersant |
| Tween 20 |
10 mg/mL |
Water |
| Tween 20 |
25 mg/mL |
Water |
| Tween 20 |
50 mg/mL |
Water |
| SDS |
25 mg/mL |
Water |
| SDS |
50 mg/mL |
Water |
Results and Discussion
The correlation functions were calculated via the scattered signals of samples.
According to the correlation functions shown above, all the samples decayed very quickly. This was because of the rapid Brownian motions of small particles.
As exhibited in Figure 1, the signal-noise ratios of correlation functions were good, and since the micelles diffused faster at higher temperatures, the decay rates increased with the temperature.

Figure 1. Correlation functions of 10 mg/mL Tween 20 at temperatures ranging from 5℃ to 65℃. Image Credit: Bettersize Instruments Ltd.

Figure 2. The overlap of the correlation function of 25 mg/mL Tween 20 at 25℃. Image Credit: Bettersize Instruments Ltd.

Figure 3. The overlap of the correlation function of 50 mg/mL Tween 20 at 25℃. Image Credit: Bettersize Instruments Ltd.

Figure 4. The overlap of the correlation function of 25 mg/mL SDS self-assembled micelle. Image Credit: Bettersize Instruments Ltd.

Figure 5. The overlap of the correlation function of 50 mg/mL SDS self-assembled micelle. Image Credit: Bettersize Instruments Ltd.

Figure 6. Particle size distributions of 10 mg/mL Tween 20 at the temperature from 5℃ to 65℃. Image Credit: Bettersize Instruments Ltd.

Figure 7. Particle size distributions of 25 mg/mL SDS micelle at 25℃. Image Credit: Bettersize Instruments Ltd.

Figure 8. Particle size distributions of 50 mg/mL SDS micelle at 25℃. Image Credit: Bettersize Instruments Ltd.
The powerful calculation capability of the BeNano 90 Zeta allows sufficient points on correlation functions for small particles. Figure 2 and Figure 3 show the correlation functions of 25 mg/mL and 50 mg/mL Tween 20 micelles gathered by multiple measurements, respectively.
It is demonstrated that the repeatability of measurements was very good even for numerous nanometer particles with very weak scattering intensity, showing the high stability and sensitivity of the BeNano 90 Zeta.
The Z-ave values of micelles formed by Tween 20 surfactant under different conditions were relatively stable, fluctuating between 7.3 nm and 7.6 nm in the concentration range from 10 mg/mL to 50 mg/mL at 25℃, as demonstrated in Table 2.
Table 2. Z-average sizes of micelle samples. Source: Bettersize Instruments Ltd.
| Sample Concentrations |
Temperature (°C) |
Z-ave (nm) |
| 10 mg/mL Tween |
5 |
7.12±0.41 |
| 10 mg/mL Tween |
10 |
8.11±0.17 |
| 10 mg/mL Tween |
15 |
8.14±0.12 |
| 10 mg/mL Tween |
20 |
7.86±0.01 |
| 10 mg/mL Tween |
25 |
7.56±0.07 |
| 10 mg/mL Tween |
35 |
7.16±0.25 |
| 10 mg/mL Tween |
45 |
6.96±0.08 |
| 10 mg/mL Tween |
55 |
6.88±0.15 |
| 10 mg/mL Tween |
65 |
7.24±0.06 |
| 25 mg/mL Tween |
25 |
7.41±0.10 |
| 50 mg/mL Tween |
25 |
7.30±0.14 |
| 25 mg/mL SDS |
25 |
53.03±7.23 |
| 50 mg/mL SDS |
25 |
2.21±0.1 |
When the concentration was 10 mg/mL, at the temperature ranging from 25℃ to 65℃, the Z-ave of Tween 20 sample was between 6.8 nm and 8.1 nm. The particle size highly depends on its concentration for SDS surfactant micelles.
At a concentration of 25 mg/mL, large aggregates of hundreds of nanometers formed in addition to the formation of multiple nanometer-sized micelles. The number of large micelle aggregates decreased significantly when the concentration increased to 50 mg/mL.
Acknowledgments
Produced from materials originally authored by Zhibin Guo, Shelly Zhang and Hui Ning from Bettersize Instruments Ltd.

This information has been sourced, reviewed and adapted from materials provided by Bettersize Instruments Ltd.
For more information on this source, please visit Bettersize Instruments Ltd.
