It is commonly understood that sample matrices have the potential to create barriers to analysis as part of routine Total Organic Carbon (TOC) analysis. Interferences from sample matrices have historically caused issues with calibration curve stability, with some frequently used aggressive matrices needing regular maintenance as well as weekly or even daily recalibration.
Veolia has successfully re-engineered the sample flow path and the oxidation technique, enabling the development of a robust TOC analyzer that is able to maintain a calibration curve for up to six months or longer, even when working with the most challenging brine matrices.
The Sievers InnovOx Laboratory TOC Analyzer makes use of Supercritical Water Oxidation (SCWO) – a novel wet chemical oxidation technique that makes use of both pressure and heat.
Increased pressure inside the reaction cell substantially enhances the efficiency of the oxidation process, enabling improved recovery for challenging matrices.
Unlike conventional combustion techniques, this approach entirely removes every by-product from the sample flow path between sample runs. Data presented in this article will demonstrate the long-term stability and calibration robustness of the Sievers InnovOx TOC Analyzer from Veolia.
What Does Supercritical Mean?
It was originally understood that there were just three phases of matter: solid, liquid and gas. Over time, however, ongoing scientific research identified 15 different phases of matter, with the ‘supercritical’ phase being most relevant here.
The point at which a compound changes phases is known as a ‘transition point.’ Before exploring the use of supercritical water, it is important to first outline the various phases of water.
Liquid water placed in an open container at room temperature and atmospheric pressure, which is then cooled to below 0°C, will transition from the liquid phase to the solid phase.
Should the temperature of the liquid water in the same open container be increased to above 100°C, the water will boil, prompting it to transition from the liquid phase to the gas phase.
This behavior is normal when atmospheric pressure is constantly maintained at ambient conditions. At the same weight or mass, liquid water and solid water will occupy approximately the same volume at atmospheric pressure.
However, the equivalent weight of gaseous water occupies approximately 1,600 times the volume when this is at atmospheric pressure.
A gas-tight lid placed on the container and an increase in temperature to 130°C would see the liquid water transition into the gas phase, but because the volume required for expansion has been limited, pressure in the headspace of the sealed container would start to increase as increasing amounts of gas are formed.
Figure 1 shows the phase diagram of water, highlighting that an increase in head pressure would increase the temperature at which the liquid water would completely transition into the gas phase.
The liquid water can therefore be heated to a higher temperature without it boiling. In the example presented here, the pressure inside the container would be almost double the atmospheric pressure. This is similar to the environment found in a common pressure cooker.
Figure 1 also confirms that as the liquid water temperature continues to increase, the corresponding pressure necessary to keep the water in a liquid phase is also greater.
There comes a point whereby it is no longer possible to increase pressure to maintain the liquid phase. Once temperature and pressure increase to more than 374°C and 218 atm (3200 psi), the water’s gas and liquid phases will combine to form another phase of matter - Supercritical Water (SCW).
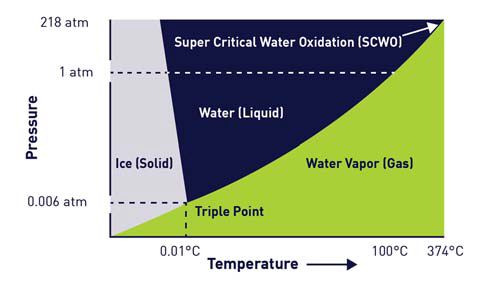
Figure 1. Phase Diagram of Water. Image Credit: Veolia Water Technologies & Solutions
Water displays the characteristics and benefits of both a liquid and a gas when it is in a supercritical state. SCW’s density is closer to that of a liquid while still being able to diffuse like a gas.
Both organic material and gases are highly soluble when immersed in the SCW, while inorganic salts become insoluble. These conditions are ideally suited for Supercritical Water Oxidation (SCWO) reactions (Figure 2).
Super Critical Water Oxidation (SCWO)
Organic + S2O8-2 + heat + Pressure → CO2 + H2O + SO4-2
Figure 2. Supercritical Water Oxidation
What is SCWO and How Does this Help TOC Analysis?
A number of techniques can be used as part of TOC measurements in order to oxidize organic carbon in the sample, therefore forming carbon dioxide (CO2).
While CO2 can be detected and quantified once it has been formed, the primary barrier to effective TOC analysis is the challenge in ensuring efficient oxidation of organic carbon.
The Sievers InnovOx TOC Analyzer employs the wet chemical oxidation technique to help mitigate this issue. This method uses an oxygen donating reagent to seed the solution, with the Sievers InnovOx making use of a 30% weight/volume solution of sodium persulphate as the oxidizer.
The sample and oxidizer are then heated past the critical point in a sealed reactor, achieving SCWO.

Figure 3. Sievers InnovOx On-Line, Lab TOC Analyzer and Autosampler. Image Credit: Veolia Water Technologies & Solutions
Research conducted in the 1970s revealed that the SCWO method offers a range of characteristics, making it ideal for analytical techniques that depends on efficient sample oxidation.
A range of studies has confirmed that this process can achieve oxidation efficiencies of more than 99% at residence times of between 10 and 30 seconds.
While the technique showed considerable promise, many hurdles had to be overcome before it was suitable for commercial use. One key challenge involved reaching the pressures and temperatures necessary for the creation of supercritical water - 218 atm (3200 psi) and 374°C, respectively.
An instrument must also be able to accommodate the powerful SCWO behavior along with any destructive sample byproducts, such as inorganic salts and acids.
The Sievers product line from Veolia represents the first successful build of a SCWO-based TOC analyzer that is suitable for commercial applications working with aqueous samples.
Through a powerful combination of a standardized wet chemical oxidizing technique and SCWO, TOC analysis has been able to reach new levels of performance criteria that remain unmatched by comparable techniques.
Instrumentation
The Sievers InnovOx Laboratory TOC Analyzer (Figure 3) operates via a simple three steps process:
- Sample handling and reagent mixing
- SCWO reaction
- Non-dispersive infrared (NDIR) detection
Each of these steps is discussed below, using Non-Purgeable Organic Carbon (NPOC) analysis as an example.
Sample Handling and Reagent Mixing
Numerous functions are automated via the sample handling module. These include sample delivery from the vial to the mixing chamber and dilution capabilities, and if required, acid addition, oxidizer addition or mixture delivery to the reaction module.
SCWO Reaction
Sodium persulphate becomes increasingly effective in oxidizing organic carbon as the temperature is applied. Until very recently, wet chemical oxidation chambers have operated at approximately 100°C, depending on the heat source in use.
Heat assistance could not be increased beyond this point because limited technology was available to effectively accommodate the increased pressure triggered by the closed vessel heating.
The Sievers InnovOx makes use of an oxidation chamber that is able to withstand these excessive pressures with ease.
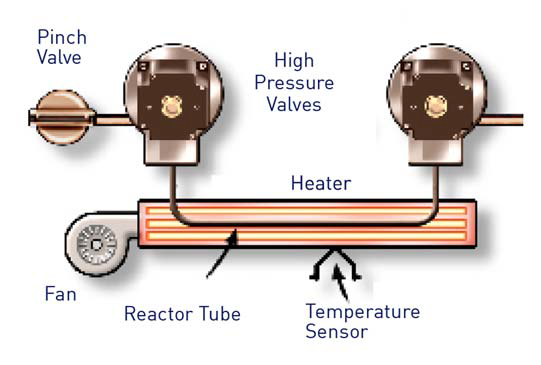
Figure 4. Sievers InnovOx Reactor Tube. Image Credit: Veolia Water Technologies & Solutions
The sample and oxidizer are delivered to the reaction module before being heated to 375°C. This maximizes the wet chemical oxide reaction, therefore allowing the water to achieve its supercritical state.
These enhanced reaction conditions lead to increased confidence in complete oxidation, irrespective of sample matrix or impurity interference (Figure 4).
NDIR Detection
Once it has been completely oxidized, the sample will be transferred to a gas/liquid separator. The isolated carbon dioxide is then passed to a calibrated non-dispersive infrared (NDIR) detector to facilitate quantification (Figure 5).
The NDIR detector employed in the Sievers InnovOx is able to offer a dynamic linearity range from 0.05 ppm to 50,000 ppm. A total of five ranges have been optimized in this instrument: up to 100 ppm, up to 1,000 ppm, up to 5,000 ppm, up to 20,000 ppm and up to 50,000 ppm.
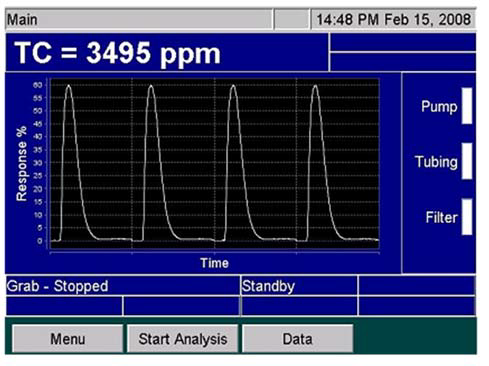
Figure 5. Peaks on the Sievers InnovOx Screen. Image Credit: Veolia Water Technologies & Solutions
Calibration - Sample Preparation
A study was conducted using up to 1,000 ppm calibration range in order to effectively demonstrate the effectiveness of SCWO. A KHP standard was created at a total carbon concentration of 1,000 ppm, and a calibration was completed using four points.
Each of these points was analyzed with nine replicates. The first three analyses were discarded before an average was taken from the remaining results (Figure 6).
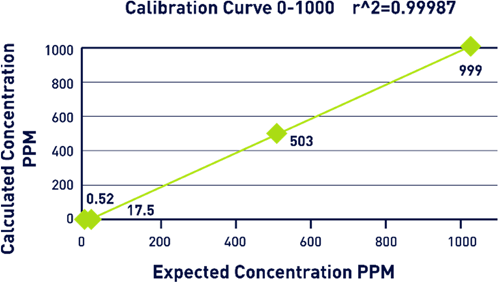
Figure 6. Calibration Curve Graph. Image Credit: Veolia Water Technologies & Solutions
Results
Verification of the calibration curve was completed using a 5 ppm stock solution that was run continuously a total of 157 times. The mean result was 5.03 ppm, while the standard deviation was 0.15, and the RSD was 3.04% (Figure 7). In total, this test represented approximately 13 hours of continuous analysis.
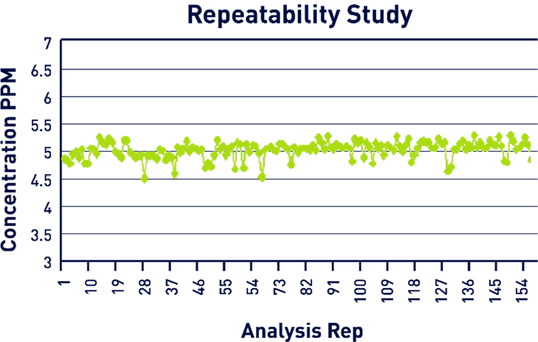
Figure 7. Repeatability Study Results. Image Credit: Veolia Water Technologies & Solutions
Conclusion
The fundamental supercritical properties of water can be leveraged through wet chemical oxidation to allow for more robust and reliable oxidation than the traditional combustion technique.
The combination of 375°C and 218 atm (3200 psi) has enabled the process to accomplish the ultra-efficient conversion of organic carbon to carbon dioxide.
By effectively managing the purging of reaction by-products and matrix impurities between each individual analysis, the InnovOx offers never-before-seen, long-term system integrity.
Each analysis commenced with a clean sample path, ensuring accurate data, robust calibration and increased time between necessary routine system maintenance.
The data shown here confidently demonstrates the advantages of enhanced wet chemical oxidation, along with the methodology’s capacity to perform effective TOC analysis on what had historically been considered very challenging or even impossible sample matrices.

This information has been sourced, reviewed and adapted from materials provided by Veolia Water Technologies & Solutions.
For more information on this source, please visit Veolia Water Technologies & Solutions