Diet is vital for our health. There has been a growing interest in food additives and dietary supplements like prebiotics like β-galactooligosaccharides (GOSs) in the past few years. Establishing the total GOS contents in food and supplements is vital to fulfilling strict food safety and labeling requirements.

Image Credit: Metrohm AG
The most widely utilized technique for total GOS determination is based on enzymatic hydrolysis to break down the complex molecules into simple carbohydrates before their chromatographic analysis.
This article describes the benefits of utilizing an improvement to AOAC Method 2001.02 by employing ion chromatography with amperometric detection (IC-PAD) and full sample automation after enzymatic hydrolysis.
What are GOSs?
GOSs are chains of galactose units with an optional glucose end. They are usually naturally present in small amounts in various beverages and foods. GOSs are added as a prebiotic supplement to infant formulas as they were first discovered as major constituents of human breast milk (present up to 12 g/L).
They exhibit bifidogenic effects, which means they support the well-being and growth of non-pathogenic gut bacteria. GOS supplements are available either raw or as concentrated syrups or powders and are utilized by food manufacturers to enrich consumer products.

Image Credit: Metrohm AG
GOS Labeling Requirements
The ongoing growth of worldwide prebiotic and GOS markets is due to increasing consumer awareness regarding healthy eating. In the same way, increased demand regarding food quality has resulted in more comprehensive, stricter rules for food labeling and safety (e.g., EU 1169/2011 and EU 2015/2283).
To fulfill such requirements, establishing the total GOS contents in supplements, food, or raw products is crucial. Studies regarding GOS health effects recommend maximum doses of less than 30 g per day, though this is much stricter for infant formulas. Otherwise, there are no other limits for GOS content in food or as nutritional supplements.
AOAC 2001.02
The standard method AOAC 2001.02 is The most widely employed technique to measure total GOSs in food products. This technique is based on the extraction of GOS from a sample followed by enzymatic hydrolysis of the oligosaccharides into monosaccharides and their subsequent analyses with high performance anion exchange chromatography with pulsed amperometric detection.
In AOAC, chromatography for anions is referred to as HPAEC (high performance anion exchange chromatography). This can be simplified to the generic term of IC. The comparison of a control solution with one which has been treated and hydrolyzed with an enzyme (β-galactosidase) is the key to AOAC 2001.02.
The enzyme catalyzes the splitting of glycosidic bonds and hydrolyzes GOSs and lactose into glucose and galactose. In order to calculate the total GOSs, the concentration differences of free galactose and lactose established in these two solutions are employed, as seen in Figure 1.
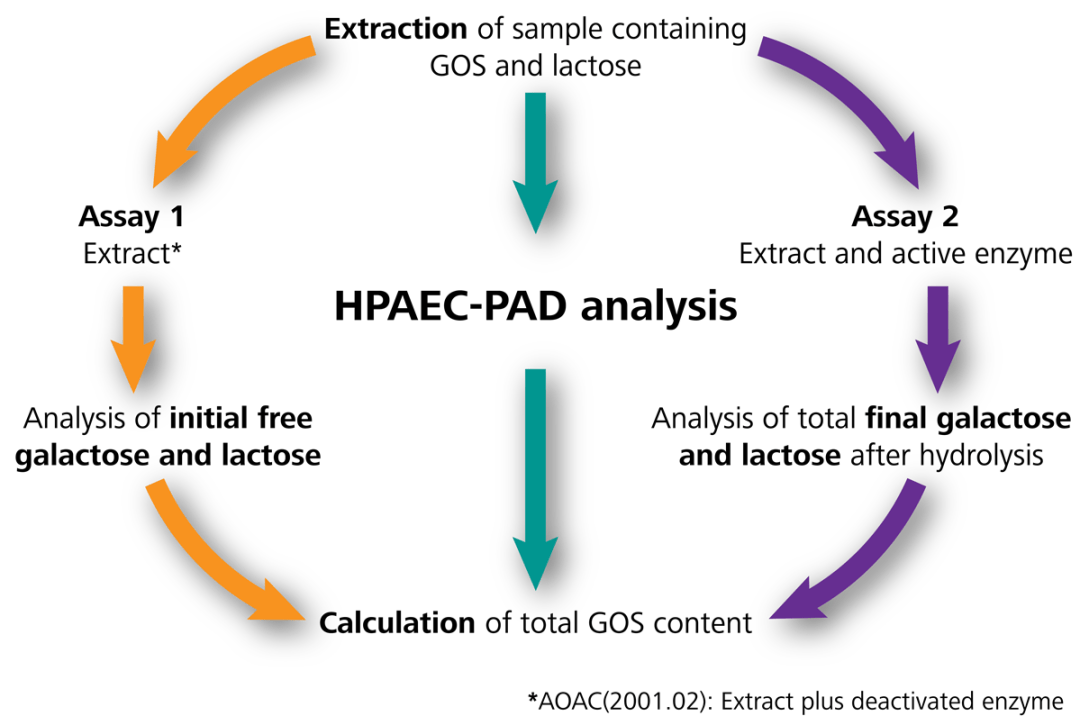
Figure 1. Schematic for determination of total GOS contents using ion chromatography with pulsed amperometric detection (IC-PAD) according to AOAC 2001.02, and an optimized method from Metrohm (in green). Chromatography for anions in AOAC is referred as HPAEC (high performance anion exchange chromatography) but is simplified here to the generic term of IC. Image Credit: Metrohm AG
Improvements to the AOAC Method
The sample preparation for AOAC 2001.02 is quite complicated: one drawback is the incubation of the reference solution with the deactivated enzyme, which can be costly, to establish the initial carbohydrate concentrations (Figure 1) instead of utilizing the pure extract.
The sample dilution procedure is another key point. While standards are based on ultrapure water, the procedure should be performed in acetonitrile. The focus here was to simplify the entire procedure to enhance the ease of use and the overall efficiency of the technique.
The improved technique for total GOS content analysis utilizes the extract for measuring the initial galactose, glucose, and lactose concentrations, as seen in Figure 1 Assay 1. Yet, the deactivated enzyme was not used, and instead, comparisons were made to see if its presence had any influence on the results.
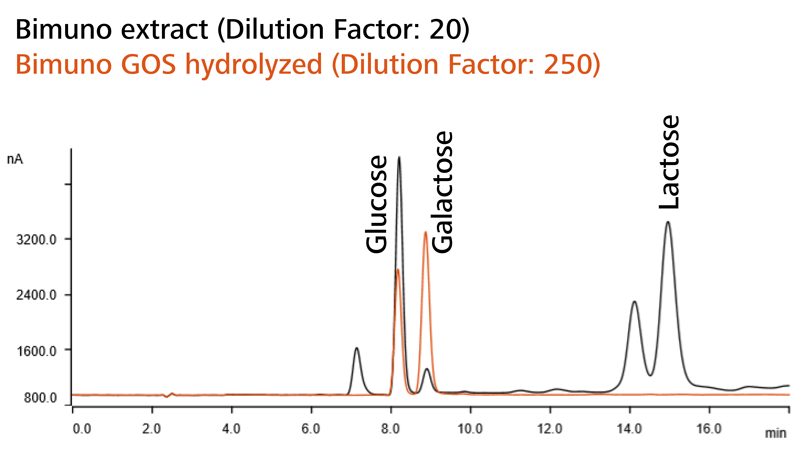
Figure 2. Overlaid chromatograms of Bimuno (prebiotic supplement), untreated (black) and treated with enzyme (orange). Image Credit: Metrohm AG
After proving results equivalent to AOAC 2001.02 Assay 1 (with the deactivated enzyme), this step was eliminated, but additional manual work and chemical expenses are reduced. As seen in Figure 2, the total GOS content is calculated from the analyte concentrations in Assay 1 (without any enzyme) and Assay 2 (extract with the active enzyme).
Other than the enzyme usage, the official AOAC technique for analysis of total GOSs indicates that standards be prepared in ultrapure water (UPW) while samples should be diluted with 20% acetonitrile. A control experiment was carried out to compare results between:
- Dilutions in UPW evaluated with UPW calibration ('UPW option')
- Dilutions in acetonitrile evaluated with UPW calibration (AOAC 2001.02)
- Dilutions in acetonitrile evaluated with acetonitrile calibration ('ACN option')
Among the three options, the reproducibility of total GOS contents was compared and the UPW and AOAC preparation options showed similar results. The ACN option resulted in lower total GOS contents.
The acetonitrile did not seem to supply a stabilizing effect to the samples. This supports the optimization of the AOAC technique by carrying out sample dilutions with UPW instead of acetonitrile, limiting the chemical imprint of the analysis and saving unnecessary reagents.
Results
Overall, together with the interference tests, the modified technique was proven as valuable and robust by the satisfying variability, target and spike recoveries. Even low total GOS contents can be determined with high precision with limits of detection (LODs) of 0.1 mg/L (galactose) and 0.2 mg/L (glucose, lactose) in solution.
Summary
As a multicomponent technique, ion chromatography with amperometric detection is an extremely sensitive, selective and robust analysis technique for carbohydrates without any further derivatization steps.
Even more complex carbohydrates can be quantified in combination with enzymatic treatment. This research demonstrates an update to the standard AOAC technique for determining total GOS in foodstuffs.
Analytical method efficiency was improved in favor of running costs and laboratory time with the same principle (enzymatic hydrolysis of complex GOS molecules followed by chromatographic analysis of simple carbohydrates). The method efficiency can be further improved by additional automation steps (e.g., Metrohm Inline Dilution and automatic calibrations).
Acknowledgments
Produced from materials originally authored by Dr. Alyson Lanciki from Metrohm

This information has been sourced, reviewed and adapted from materials provided by Metrohm AG.
For more information on this source, please visit Metrohm AG.