In this interview, Dr. James White, P.h.D, the Founder of Applied Extracts, talks to AZoM about the advantages of Supercritical CO2.
To begin, can you give us an introduction into supercritical CO2– what is it, and what does it involve?
Supercritical CO2 is simply carbon dioxide that is pressurized and heated above its critical point (31.1 oC, 1081 psi). The fluid has many useful properties including low viscosity, high density, very low cost and leaves no residual solvents. The carbon dioxide used in supercritical CO2 processes is collected from other large-scale industrial processes and is merely diverted through the extraction or separation equipment. Supercritical CO2 is used in many green chemistry processes due to its neutral environmental footprint.
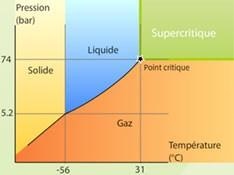
Image Credit: Le Portail des Fluides Supercritiques
Supercritical CO2 is an extremely popular solvent for industrial processes, due to it being so cheap, but also because it is not toxic, and not considered a ‘volatile’ solvent. For these reasons, Supercritical CO2 is described as “Generally Regarded as Safe” (GRAS), which is an important classification when considering industrial applications.
What are some of the advantages of supercritical CO2, and on the other hand, what are some of the challenges that come alongside it?
Carbon dioxide has the advantage of being non-flammable and non-toxic, which is not generally true of other common solvents. The disadvantage of this solvent is that it requires relatively high pressure compared to hydrocarbon solvents. A good quality supercritical CO2 extraction machine, for example, will operate at 3800 psi or higher. High-pressure machinery can operate at 15000 psi or greater. A lot of engineering and expensive machinery is needed to generate and control these high pressures. The benefit is that the solvent cost is a fraction of hydrocarbon solvents and there is no need to operate in a hazardous location rated environment.
In addition to being GRAS (Generally Regarded as Safe) as well as it being so inexpensive, supercritical CO2 has many advantages with regards to its ‘tunability’, meaning that incremental changes to temperature and/or pressure, the ‘behavior’ of the solvent can be highly selective when used for extractions and separations. One particular challenge with Supercritical CO2 is that, due to its relatively high operating pressures, there may be some ‘unwanted’ extraction/separation results. For example, with botanical extractions, Supercritical CO2 extracts many fats & lipids from botanical matter, which, in general, are not desirable.
What are some of the easier ways to determine whether you should employ supercritical or subcritical methods – what is the difference?
“Sub-critical” simply refers to a state of matter that is below the critical point. For carbon dioxide, this could be solid, liquid or gas phase. For extractions, liquid phase carbon dioxide is commonly used since it operates at lower temperatures. The disadvantage is that the solubility of liquid carbon dioxide is orders of magnitude less than supercritical carbon dioxide. The only way to know with certainty whether supercritical or subcritical carbon dioxide is the preferred method is to run the experiments and collect data. We offer process development services to our clients on a fee basis.
Easy ways to determine whether or not Supercritical CO2 is right for you to include answering the following questions:
- What is the application? Supercritical CO2 is not an effective solvent for all separations, however, the list of effective applications is extensive.
- What is feasible for your facility/laboratory? Some labs prohibit volatile solvents like alcohols or LPG (Liquid Petroleum Gas), which make supercritical CO2 a viable option for even more labs.
- What specific products are you making? Some products are simply more desirable to utilize subcritical CO2, although the extraction process is generally very slow compared to Supercritical CO2.
Can you tell us about some of the different supercritical CO2 applications in extraction?
For small-scale extraction, the primary application in the last ten years has been the extraction of cannabinoids from hemp and marijuana. The cannabis market has given rise to many small-scale operators who process a few pounds every hour or even a few pounds per day. This market for small-scale machinery previously only existed in academic and pharmaceutical research applications.
Large scale industrial users of supercritical carbon dioxide perform an immense variety of extractions, including hops, decaffeination of green coffee beans, chili peppers, black pepper (organic dyes), rye pollen (dietary supplements), algae, aerogels (advanced materials), palm oil, monodisperse powders (pharmaceutical applications), and dozens of other commercial uses.
In terms of botanical applications, what are some of the latest trends in terms of supercritical CO2?
The big story in botanical oils, since 2010, has been cannabis extractions. Supercritical CO2 is highly competitive with butane and ethanol extractions, particularly for large-scale industrial extraction facilities that are found in the hemp industry. At a large scale, solvent costs dominate the operating cost of the extraction facility and that is where supercritical CO2 excels. Bulk carbon dioxide costs $0.06-$0.10 per pound; no hydrocarbon or alcohol-based solvent can come close to that cost.
Supercritical CO2 has become a favorite of eco-conscious producers of essential oils, cannabis & hemp extracts, and nutraceuticals due to its Green properties. In addition to these environmental benefits, regulatory bodies (state, federal, local) are also much more willing to issue/grant permits to these types of businesses, due to safety. More and more processors are choosing to center their businesses around CO2 as a result.
At Applied Extracts, you are currently investigating aerogel drying with supercritical CO2 – can you tell us more about this?
Aerogels are used commercially in aerospace and space applications. Innovative manufacturers are finding ever more uses for aerogels in other application areas requiring a lightweight, strong mechanical structure. In this case, we are investigating aerogel drying of a novel compound on behalf of a client. If successful, the project would move to a pilot plant scale and then full-scale production.
How effective is supercritical CO2 in terms of sterilization – what are some of the latest advancements surrounding this?
In the sterilization process, supercritical CO2 is combined with hydrogen peroxide as a co-solvent. The combination of fluids is a highly effective sterilization technology – sterilization as low as SAL 6 can be obtained. The intent of this development is to replace ethylene oxide and gamma radiation as sterilization methods. The technique is particularly applicable to materials that cannot withstand the temperatures found in an autoclave, and in instances where gamma irradiation or ethylene oxide are not feasible due to cost, logistics or other considerations. There are some limitations on compatible materials, however- for example, some rubber compounds are permeable to supercritical carbon dioxide and can be damaged by rapid depressurization.
With the growing advancements in technology, how much has this changed the industry, and where do you think the industry is heading?
The fundamental technology of supercritical carbon dioxide is very old. The technology has existed for over a century. The main driving force for wider adoption of supercritical carbon dioxide processing methods is environmental; procurement and disposal costs for hydrocarbon solvents are constantly rising, occupational safety and hazardous materials handling are expensive, and supercritical carbon dioxide can often alleviate or eliminate these costs.
Where can readers find more information?
There are hundreds of excellent peer-reviewed journal articles and references available in the literature. For people interested in learning more about applications of supercritical carbon dioxide extractions, I always recommend the following book:
“Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds” Edited by Jose L. Martinez, 2007.
Readers can also look beyond supercritical carbon dioxide at processes involving other supercritical fluids – supercritical water is one of the more interesting possibilities for green chemistry processes. There are many new processes still to be discovered in the field.
About Applied Extracts
Applied Extracts was founded in 2017 by Dr. James White, Ph.D. We manufacture high-pressure supercritical carbon dioxide extraction equipment for the nutraceutical industry. Applied Extracts also provides green chemistry research and development services for its clients.
About Dr. James White, Ph.D. 
Dr. White is a lifelong engineer and entrepreneur with a successful track record of developing and commercializing high-performance scientific instruments and pharmaceutical production equipment. Dr. White graduated from the Massachusetts Institute of Technology in 2003, with a doctorate in Mechanical Engineering.
Disclaimer: The views expressed here are those of the interviewee and do not necessarily represent the views of AZoM.com Limited (T/A) AZoNetwork, the owner and operator of this website. This disclaimer forms part of the Terms and Conditions of use of this website.