The use of antibiotics in animal husbandry is carried out to prevent or treat diseases in livestock and increase productivity.
The widespread practice of using antibiotics in animals can have several consequences, including:
- Incidents of food contamination
- Causing environmental residues
- Adverse health effects in humans
- The occurrence of bacterial strains that are resistant to antibiotics
In the apiculture sector, sulfonamides are the most frequently employed antibiotics in the treatment of American and European Foulbrood (a disease that infects bees).
Foulbrood can lead to bee honey becoming contaminated with sulfonamides. For this reason, it is imperative to develop a method for quantitating sulfonamides in honey that is quick, consistent and vigorous. This article outlines such a method.
Sample Preparation
The sample was extracted and cleaned up as follows:
- A honey sample weighing 5 g was placed in a 150 ml beaker.
- A 25 ml measure of phosphoric acid solution with a pH of 2.0 was added.
- The resulting solution was vortexed and mixed until the honey was thoroughly dissolved.
The sample clean-up involved:
- The dissolved sample was loaded onto a preconditioned aromatic sulfonic cation-exchange cartridge at a flow rate of ~2 ml/min.
- The cartridge was washed with 5 ml of phosphoric acid solution with a pH of 2, and 5 ml of water.
- A 40 ml measure of potassium phosphate buffer (0.2 mol/L, pH=8) was utilized to elute the analytes from cartridge.
- A 1.5 ml measure of sodium heptanesulfonate solution (0.5 mol/L) was added to the eluent.
- The pH was adjusted to 6 by the addition of phosphoric acid.
- The resulting solution was then loaded onto a HLB cartridge (which had been preconditioned) at a flow rate of ~2 ml/min.
- The cartridge was washed with 3 ml of water and dried under vacuum conditions.
- The analytes were eluted from the cartridge by using 10 ml of methanol.
- The eluent was evaporated to dryness and the residue dissolved in 1 ml of mobile phase A.
Experimental
The Hardware and Software
A PerkinElmer QSight® 210 UHPLC-MS/MS system was utilized to carry out the separation and detection of the analytes. The Simplicity 3Q™ software platform was employed for all instrument control, as well as the analysis and data processing procedures.
Method Parameters
The method parameters for LC and MS/MS can be seen in Tables 1 and 2, respectively.
Table 1. LC method parameters. Source: PerkinElmer Food Safety and Quality
| . |
. |
| Mobile Phase: |
Solvent A: Water containing 0.1% of formic acid (FA)
Solvent B: Acetonitrile (ACN)
| |
Time (min) |
Flow rate (mL/min) |
%A |
%B |
Curve |
| 1 |
Initial |
0.4 |
90 |
10 |
| 2 |
1.00 |
0.4 |
90 |
10 |
6 |
| 3 |
4.00 |
0.4 |
70 |
30 |
6 |
| 4 |
5.00 |
0.4 |
70 |
30 |
6 |
| 5 |
6.00 |
0.4 |
10 |
90 |
6 |
| 6 |
6.50 |
0.4 |
10 |
90 |
6 |
| 7 |
7.00 |
0.4 |
90 |
10 |
6 |
| 8 |
9.00 |
0.4 |
90 |
10 |
6 |
|
| Oven Temp.: |
40 ºC |
| Injection Volume: |
3 μL |
Table 2. MS/MS parameters and retention times of the analytes. Source: PerkinElmer Food Safety and Quality
| Ion source |
ESI Positive |
| ElectroSpray/V |
4500 |
| Heating Gas Temp/°C |
500 |
| HSID Temp/°C |
320 |
| Dry Gas |
200 |
| Nebulizer Gas |
180 |
| Analytes |
RT |
Precursor ion/product ion 1 |
EV (entrance voltage) |
CC (collision energy) |
Precursor ion/product ion 2 |
EV |
CC |
| Sulfadiazine |
1.34 |
251.2/156.1 |
28 |
-21 |
251.2/92.2 |
22 |
-40 |
| Sulfathiazole |
1.63 |
256.1/156.1 |
26 |
-20 |
256.1/108.1 |
25 |
-40 |
| Sulfapyridine |
1.77 |
250.2/156.1 |
24 |
-24 |
250.2/184.1 |
28 |
-24 |
| Sulfisoxazole |
2.65 |
268.1/156.1 |
27 |
-21 |
268.1/113.2 |
26 |
-29 |
| Sulfamethizole |
2.86 |
271.1/156.1 |
19 |
-20 |
271.1/108.0 |
25 |
-38 |
| Sulfamethazine |
3.02 |
279.2/186.1 |
18 |
-24 |
279.2/156.1 |
18 |
-25 |
| Sulfachloropyidazine |
3.66 |
285.1/156.1 |
23 |
-23 |
285.1/108.1 |
21 |
-37 |
| Sulfamethoxazole |
3.79 |
254.2/156.1 |
29 |
-23 |
254.2/108.1 |
28 |
-36 |
| Sulfamonomethoxine |
4.15 |
281.0/155.8 |
19 |
-24 |
281.0/108.0 |
30 |
-38 |
| Sulfadimethoxine |
5.11 |
311.3/156.1 |
35 |
-27 |
311.3/218.1 |
24 |
-26 |
| Sulfaquinoxaline |
5.18 |
301.1/156.1 |
22 |
-23 |
301.1/108.0 |
18 |
-40 |
Results
The total ion chromatogram (TIC) of 11 sulfonamides analyzed in MRM transition mode are shown in Figure 1.
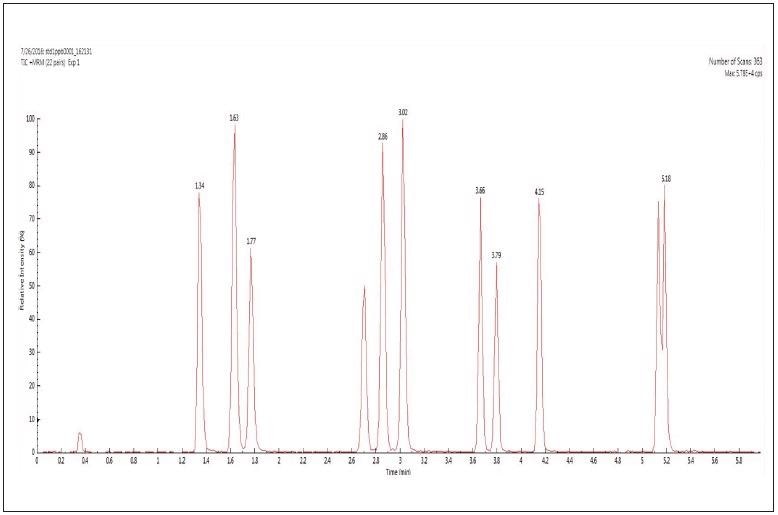
Figure 1. TIC of 11 sulfonamides analyzed in MRM transition mode. Image Credit: PerkinElmer Food Safety and Quality
Summaries of the signal-to-noise ratios (obtained at 0.02 ng/ml), and recoveries of the individual sulfonamides spiked in honey brought through the complete extraction procedure can be found in Table 3.
Table 3. Signal-to-noise ratio of the analytes at 0.02 ng/mL, and recovery of the analytes from sample at 0.02 μg/kg. Source: PerkinElmer Food Safety and Quality
| Analytes |
S/N at 0.02 ng/mL |
Recovery at 0.02 μg/kg (%) |
| Sulfadiazine |
18 |
81.6 |
| Sulfathiazole |
40 |
85.9 |
| Sulfapyridine |
30 |
83.2 |
| Sulfisoxazole |
50 |
86.2 |
| Sulfamethizole |
100 |
90.1 |
| Sulfamethazine |
400 |
91.5 |
| Sulfachloropyidazine |
30 |
79.8 |
| Sulfamethoxazole |
30 |
81.1 |
| Sulfamonomethoxine |
20 |
77.5 |
| Sulfadimethoxine |
25 |
78.0 |
| Sulfaquinoxaline |
40 |
80.7 |
Table 4. Linear dynamic range, regressional coefficients, LOQ and reproducibility at LOQ. Source: PerkinElmer Food Safety and Quality
| Analytes |
Linear dynamic range |
Regressional coefficients (R2) |
LOQ (ng/mL) |
CV% at LOQ n=6 |
| Sulfadiazine |
0.01~10.0 |
0.9943 |
0.01 |
5.41 |
| Sulfathiazole |
0.01~10.0 |
0.9950 |
0.01 |
6.25 |
| Sulfapyridine |
0.01~10.0 |
0.9936 |
0.01 |
4.77 |
| Sulfisoxazole |
0.01~10.0 |
0.9958 |
0.01 |
4.90 |
| Sulfamethizole |
0.01~10.0 |
0.9943 |
0.01 |
3.22 |
| Sulfamethazine |
0.01~10.0 |
0.9950 |
0.01 |
2.15 |
| Sulfachloropyidazine |
0.01~10.0 |
0.9948 |
0.01 |
6.54 |
| Sulfamethoxazole |
0.01~10.0 |
0.9934 |
0.01 |
7.03 |
| Sulfamonomethoxine |
0.01~10.0 |
0.9972 |
0.01 |
6.05 |
| Sulfadimethoxine |
0.01~10.0 |
0.9958 |
0.01 |
7.12 |
| Sulfadiazine |
0.01~10.0 |
0.9959 |
0.01 |
5.79 |
The quantitating of the sulfonimides present in the honey was done by employing external standard calibration curves. These were generated by dissolving standards at 0.1-10 ng/ml in 90% water/ACN.
The findings indicate very good linearity within the concentration range, with regression coefficients of (R2) ≥ 0.99. The limit of quantitation (LOQ) was 0.01 ng/ml for every analyte. There was good reproducibility (CV<8%).
Conclusion
A quick and reliable UHPLC-MS/MS method was developed for the determination of 11 sulfonamides in honey. The extraction and clean-up sample preparation method revealed good recoveries (>80%) for every analyte in the sample. The LOQ for each analyte was 0.01 ng/ml (0.01 µg/kg).
A maximum concentration level of 50 µg/kg for sulfonamides in honey was recommended in an EU Community Reference Laboratories' (CRLs) guidance paper.
The LOQs attained using the method outlined here are well beneath that level. This suggests that the QSight 200 System offers a platform for the analysis of sulfonamides in honey that is both robust and sensitive.
Acknowledgments
Produced from materials originally authored by the Mass Spectrometry Team at PerkinElmer Inc.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Food Safety and Quality.
For more information on this source, please visit PerkinElmer Food Safety and Quality.