Apple juice is considered a healthy alternative to artificially flavored and carbonated drinks, making it a preferential choice for many parents to give to children and posing as a valuable source of vitamins, minerals, and fiber.1
However, some concern has been expressed over the presence of arsenic in apple juices2 due to naturally high levels and the historical use of arsenic in pesticides, where arsenic from such applications can remain in the soil for decades after its use.
This is of particular concern in exposures involving children since children suffer a more extreme dose-response than adults.3
While the information acquired from the complete analysis of arsenic can be useful, it may also produce a biased view about toxicity since various chemical forms of arsenic have different levels of toxicities.
Comparatively speaking, speciation studies have the capacity to offer a wealth of information regarding toxicity as this is relative to chemical species present and the abundance of each relevant species.
For arsenic, the most toxic forms are the inorganic forms (arsenite [As III] and arsenate [As V]), whereas methylated forms of arsenic (dimethylarsinic acid [DMA], monomethylarsonic acid [MMA], etc.) are less toxic.
As a result of this, the action limit for inorganic As in apple juice as determined by the FDA is 10 µg/L,4 as concentrations greater than this have been found to provoke a toxicological response in children.1,2,3,5
Chromatographic techniques, such as HPLC are often used to separate the various chemical species of As, where ICP-MS is the preferred detector for many analytical laboratories.
This is because it possesses a wide linear dynamic range, low detection limits, and has the capacity to resolve complex interferences.
For analytes other than As, the additional capacity of ICP-MS to resolve various isotopes of elements allows users to make use of isotope dilution methods, which can vastly improve analytical accuracy.
A challenge HPLC-ICP-MS users face is that most applications utilize salt buffers in strongly acidic6,7 or strongly basic9 mobile phases so to establish the baseline resolution of the various chemical species of the element of interest.
In steel-based systems, excessive conditions may lead to corrosion of hardware over long-term use including wear to the pump and autosampler; this effect will be even more noticeable in components that make contact with the mobile phase. While these systems can be fairly passivated by flushing with 20% HNO3, there is still potential for corrosion.10
Any rust build-up may become dislodged, sometimes blocking and irreversibly damaging expensive columns.
However, this can be prevented effectively when utilizing an HPLC system that has an inert, metal-free fluid path that would eradicate the issue of rust formation. Moreover, using this design would guarantee backgrounds are low for other analytes which in trace speciation applications are usually of analytical interest, such as Cr and Fe.
An additional hurdle for users to overcome is that in spite of good pump practice which includes flushing the pump with a weak organic solution at the end of each day, salt crystals can still build up behind the pump seals. This can lead to seal damage, impacting analytical results, thereby increasing maintenance costs and instrument downtime.
This issue can be relieved by using an HPLC pump that has post-seal wash capabilities, meaning actively washing behind the pump seal, in order to reduce seal wear and tear.
Throughout the study, characterization of four arsenic species in commercial apple juices was performed using an HPLC method which was developed to accomplish the fast and reproducible separation and analysis of low concentrations of As species.
The verified and reputable method developed by Ernstberger et al.7,8 was employed to quantify the different arsenic species typically found in apple juice (As III, As V, DMA, MMA) and thereby assess their potential toxicity.
This analysis was conducted using a PerkinElmer NexSAR™ HPLC-ICP-MS Solution, which is made up of an inert NexSAR Speciation Analysis Ready HPLC system paired with a NexION® ICP-MS, where the elution was controlled thermostatically utilizing a NexSAR Column Oven.
Experimental
Sample Preparation
Preparations of calibration standards with concentrations of 0.1, 0.5, 1, 5, 10, and 20 μg/L were carried out in the mobile phase using the following reagents: As (III) from 999 ± 5 mg/L arsenite (Inorganic Ventures Ltd., Christiansburg, Virginia, USA), As (V) from 1003 ± 6 mg/L arsenate (Inorganic Ventures), DMA from cacodylic acid (≥ 99.0%, Sigma Aldrich, St. Louis, Missouri, USA), and MMA from 1000 mg/L MMA (Chemservice, West Chester, Pennsylvania, USA).
These species were chosen for evaluation as they were shown to be present in previous past studies involving the analysis of apple juices.6,7 Concentrations were selected to reflect the concentration range as set out by the FDA around the current action level for inorganic arsenic.4
The analysis was performed using an acidic mobile phase (pH 4.0) which previously had proven to be the most appropriate method which properly reflects the pH of apple juices, while also sustaining the integrity of the various arsenic species.7 Four popular, commercially produced apple juices were bought from a local grocery store.
There was no attention given to the shelf-life of these juices. This is due to it being known that the various chemical species remain in equilibrium, and are stable in commercial-grade apple juices across prolonged periods of storage time.6,7
Juices were shaken vigorously prior to sampling to ensure homogeneity, and 50 mL of each apple juice was passed through 0.45 µm PTFE filters (hydrophilic, Millex, Sigma Aldrich) to eliminate any unwanted particulate matter.
Analyses were conducted on undiluted samples. Throughout the study, the calibration standards were continuously carried out, and the four apple juice samples were repeatedly analyzed after being poured into a number of different plastic HPLC vials.
A blank sample was evaluated after every apple juice sample to check for carryover between samples. In the absence of certified reference material, accuracy was secured by spiking the samples with 2 µg/L and 10 µg/L of each species (As III, As V, DMA, MMA).
Instrumentation
All analytical evaluations were carried out using a NexSAR Speciation Analysis Ready HPLC system (PerkinElmer Inc., Shelton, Connecticut, USA) which contained the NexSAR 200 Inert HPLC Pump, NexSAR Cooled Inert Autosampler, NexSAR Solvent Tray with Degasser, and NexSAR Column Oven (PerkinElmer Inc.).
The system was paired with a NexION ICP-MS (PerkinElmer Inc.). Details relating to the HPLC and ICP-MS conditions are exhibited in Tables 1 and 2, respectively, in accordance with previous work.7
During the development of the method, m/z 75 and 77 were monitored to keep an eye out for the presence of 75ArCl+ interference on 75As+. As no ArCl+ was detected, the samples were analyzed in Standard mode for the remainder of the study. All analyses and data acquisition was conducted using Clarity™ software version 8.1.
Table 1. NexSAR Inert HPLC System Conditions. Source: PerkinElmer Food Safety and Quality
| Parameter |
Value |
| Chromatography |
Reversed-phase Ion-pairing Chromatography |
| Mobile Phase |
Ion-pairing Reagent |
| pH |
4.0 |
| Separation Scheme |
Isocratic |
| Injection Volume |
20 μL |
| LC Vials |
HPLC Tested PP Vials, 1.5 mL |
Table 2. NexION ICP-MS Instrument Conditions. Source: PerkinElmer Food Safety and Quality
| Parameter |
Value |
| Nebulizer |
MEINHARD® plus Glass Type C |
| Spray Chamber |
Glass Cyclonic |
| RF Power |
1600 W |
| Injector |
2.0 mm ID Quartz |
| Nebulizer Flow |
Optimized for <2% oxides |
| Mode |
Standard |
| Dwell Time |
100 ms |
| Sampling Rate |
10 points/sec |
Results and Discussion
The correlation coefficients for the standards (0.1 – 20 µg/L) of As V, MMA, As III, and DMA were 0.99990, 0.99999, 0.99999, and 0.99998 respectively (Figure 1), with Figure 2 showing the intersection of the calibration standards.
The latter (Figure 2) highlights the reliable and consistent flow rate provided by the pump and demonstrates that there is exceptional reproducibility of the retention times, regardless of the concentration.
Due to the inanimate fluid path of the NexSAR HPLC system, the chromatogram baseline is insignificant for As, with the signal-to-noise (S/N) ratio for the 0.1 ppb standard ranging from 12 and 7 for the different chemical species of As.
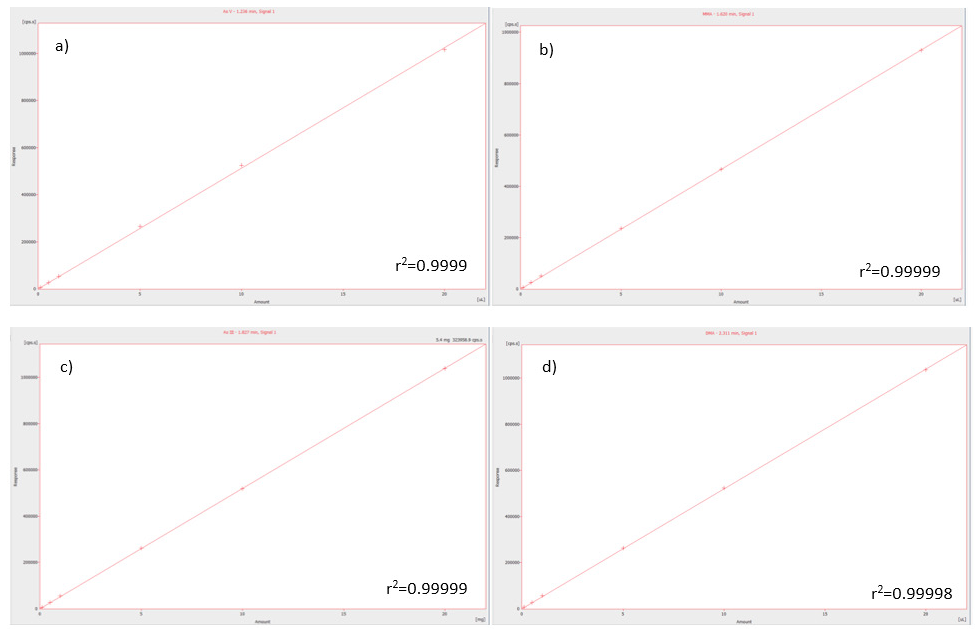
Figure 1. Linear regression of calibration standards ranging in concentration from 0.1-20 μg/L for (a) As V, (b) MMA, (c) As III, and (d) DMA in the mobile phase (pH 4.0) and the respective correlation coefficients. Image Credit: PerkinElmer Food Safety and Quality
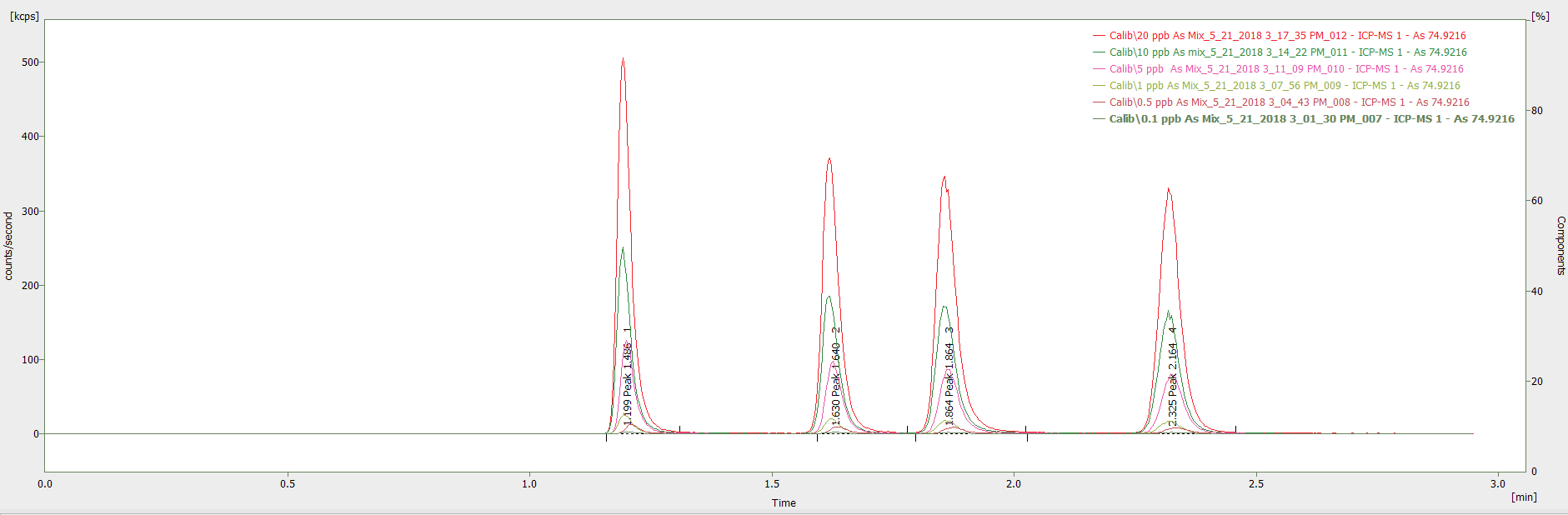
Figure 2. Chromatogram showing overlay of calibration standards (0.1 – 20 μg/L) in the mobile phase at (pH 4.0). Image Credit: PerkinElmer Food Safety and Quality
This facilitates the detection of As at concentrations lower than the FDA action limits and also ensures that chemical species, which were present in low levels – such as MMA – can be quantified easily. This is critical because detecting chemical species, which are present in low concentrations, offers a more holistic view of toxicity.
In studies where mass-balance equations are utilized, the ability to quantify trace levels of analytes would significantly improve analyte recoveries, thereby enhancing the overall quality of such results.
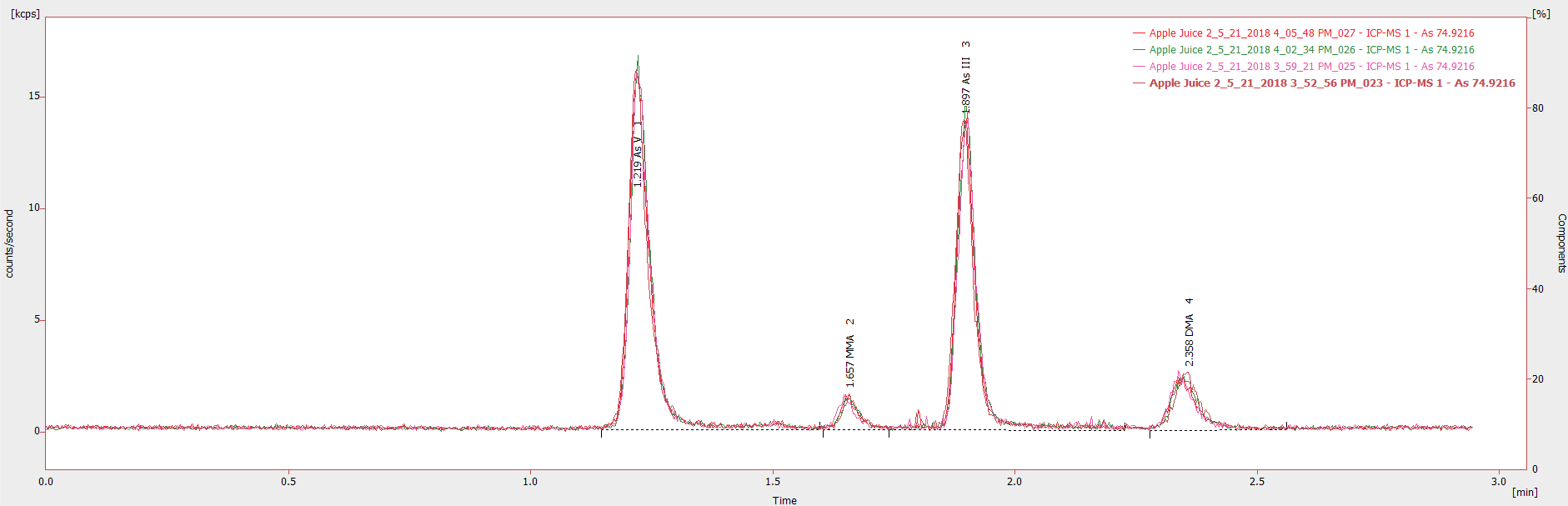
Figure 3. Chromatogram showing four injections (20 μL) of an undiluted apple juice sample from different sample vials. Image Credit: PerkinElmer Food Safety and Quality
Achieving reproducible results in undiluted samples can sometimes be challenging due to the fact apple juice is a complex matrix. During this study, injections of the same sample from various sample vials were seen to exhibit exceptional reproducibility (Figure 3) with a percentage relative standard deviation (% RSD) less than 2% for all analytes.
Low chromatographic baselines with pulsation deemed inconsequential for both the samples and calibration standards shows the strength of the technique across various matrices and the validity of the HPLC-tested plastic vials for this application.
The overlay also reveals that injection volumes were exceptionally repeatable, having a near-exact response at each injection. It should be noted that better quality solvents and chemicals could reduce the chromatographic baseline (S/N).
Regardless, Figure 3 shows that low-ppt analytes can be quantified precisely where the concentrations of As V, MMA, As III, and DMA are 0.94, 0.09, 0.77, and 0.20 µg/L respectively in this specific sample, and the S/N for the lowest concentration analyte (MMA) was 6.
Figure 3 also illustrates that of the four most frequently used and relevant arsenic chemical species detected in apple juices, complete separation and precise quantification can take place in less than three minutes per sample.
This elution time is five times faster than conventional techniques, such as anion exchange chromatography, applied in arsenic speciation studies.
This work was carried out without an internal standard and the reproducibility shown indicates that there is no need for one; however, if an internal standard is necessary previous work has demonstrated that arsenobetaine (AsB) can be applied as an appropriate internal standard eluting just after three minutes.7
The analysis of a blank after each apple juice sample demonstrated that there was no carryover between injections.
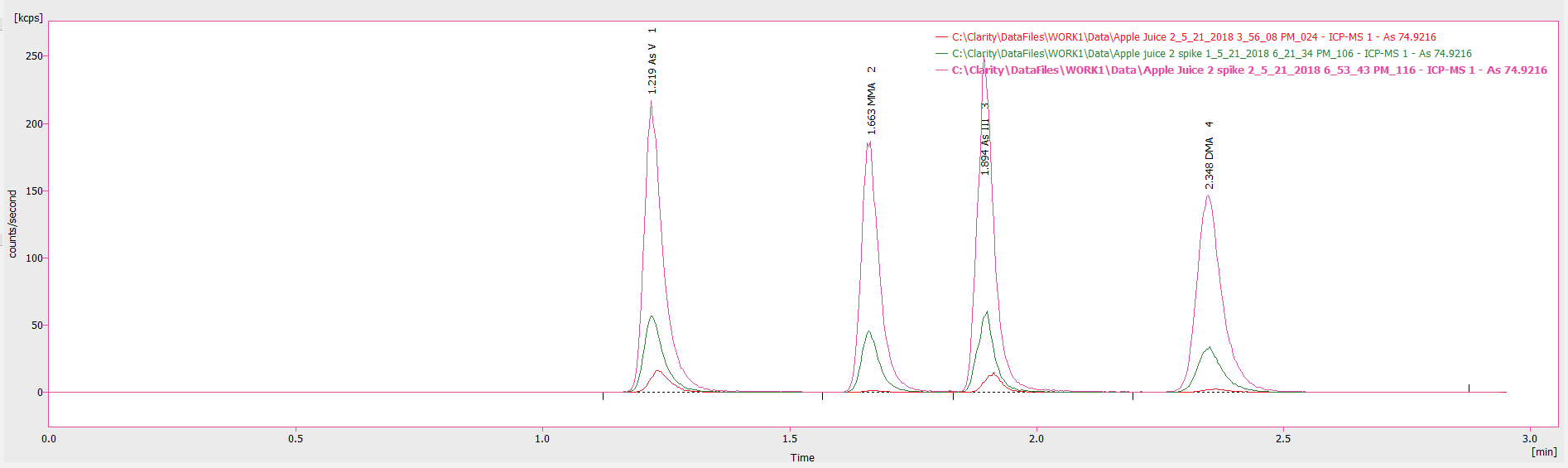
Figure 4. Chromatogram showing an undiluted apple juice sample as well as low-end (2 μg/L) and high-end (10 μg/L) spikes of this sample. Image Credit: PerkinElmer Food Safety and Quality
The absence of carryover is very useful, specifically in commercial laboratories, where limiting rinsing between samples reduces solvent use, generating higher productivity and lower overheads.
To evaluate the influence of the apple juice matrix upon analytical accuracy, a low-end (2 µg/L) and high-end (10 µg/L) spike of each arsenic species was introduced to an undiluted sample. Figure 4 illustrates how the chromatogram for the 2 µg/L and 10 µg/L spikes when compared with the sample.
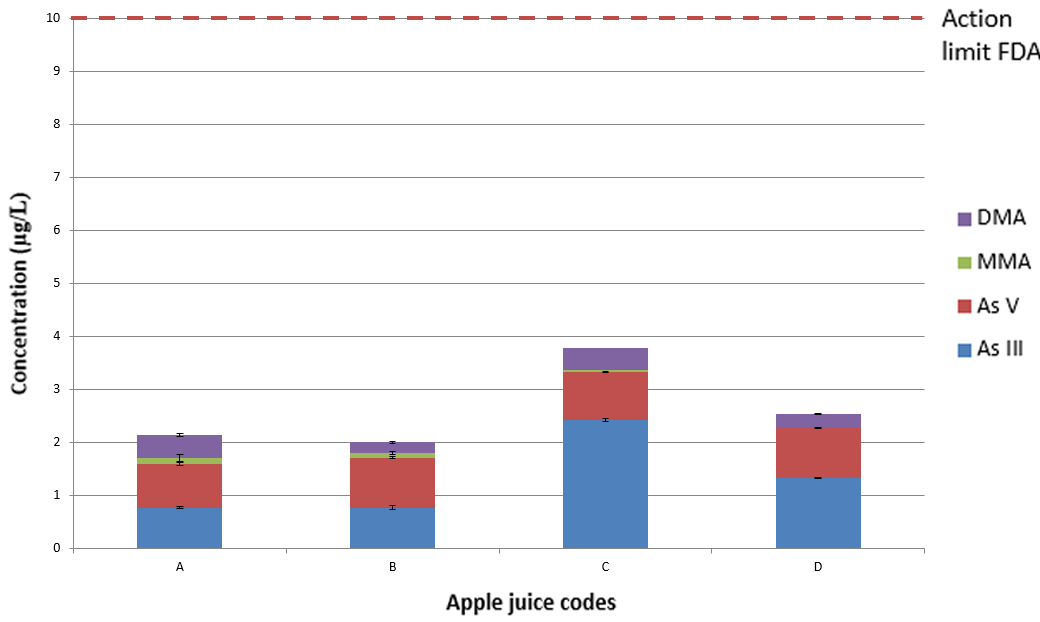
Figure 5. Averaged concentrations of As III, As V, MMA, and DMA in four commercially produced apple juices compared to the action limit of 10 μg/L, where the error bar shows the standard deviation (SD) across replicate analyses. Image Credit: PerkinElmer Food Safety and Quality
It was determined that spiked samples possess similar retention times to the pure sample and demonstrated good spike recoveries, ranging between 99-111% for the various arsenic species (2 μg/L spike: 103, 111, 100, 99% recovery and 10 μg/L spike: 106, 103, 111 and 101% recovery for As V, MMA, As III, and DMA, respectively).
This is a testimony to the accuracy of the method across a greater linear dynamic range and demonstrates that samples can be prepared in a straightforward manner.
Figure 5 exhibits the sample results collected. As determined in previous studies, the inorganic forms of arsenic are the dominant forms (As III and As V), where DMA was the third most prevalent and MMA was present in just a few samples.6,7
It is clear that each of the apple juices has various concentrations of the different chemical species of arsenic, however, all samples range between 16-34% of the action level of inorganic arsenic5 and, as a consequence, are below the FDA’s toxicological concern threshold.4
Conclusion
This study demonstrated that using the reversed-phase ion-pairing method facilitates the complete separation and precise quantification of the main arsenic species in apple juices that are commercially available, including the primary toxic forms (As III and As V), in less than three minutes.
This work was conducted using a NexSAR HPLC-ICP-MS Speciation Analysis Ready Solution, where concentrations resided in the µg/L and ng/L range.
The concentrations of inorganic As in all the apple juice samples evaluated in this study were below the FDA recommended action limit of 10 μg/L.4
The power of the suggested methodology in combination with the reliability and strength of the hardware were exhibited through repeatability, carryover, and spike recovery studies.
The inert fluid path of the NexSAR Pump and Autosampler, and the post-seal wash of the pump, offer the peace of mind that samples have the potential to routinely run at a low pH (pH 4.0 in this study). This is with both salted buffers and without being at risk for corrosion and substantial seal damage.
References
- Gerhauser C. 2008. Cancer Chemopreventive Potential of Apples, Apple Juice, and Apple Components, New York: Planta Medica DOI: 10.1055/s-0028-1088300.
- Wilson D, Hooper C, Shi W. 2012. Arsenic and Lead in Apple Juice: Apple, Citrus and Apple-Base, Journal of Environmental Health, 75: 14-21.
- de Burbure C, Buchet JP, Leroyer A, Nisse C, Haguenoer JM, Mutti A, Smerhovsky Z, Cikrt M, Trzcinka-Ochocka M, Razniewska G, Jakubowski M, Bernard A. 2006. Renal and Neurologic Effects of Cadmium, Lead, Mercury, and Arsenic in Children: Evidence of Early Effects and Multiple Interactions at Environmental Exposure Level, Environmental Health Perspective, 114:
- FDA. 2013. Supporting Document for Action Level for Arsenic in Apple Juice. FDA-2012-D-0322.
- Bhattacharya P, Welch AH, Stollenwerk KG, McLaughlin MJ, Bundschuh J. 2007. Arsenic in the Environment: Biology and Chemistry, Science of the Total Environment, 379: 109-120.
- Neubauer K, Perrone P, Reuter W. 2012. Determination of Arsenic Speciation in Apple Juice by HPLC/ICP-MS. PerkinElmer Application Note.
- Ernstberger H, Neubauer K. 2015. Accurate and Rapid Determination of Arsenic Speciation in Apple Juice, PerkinElmer Application Note.
- Ernstberger H, Neubauer K. 2015. HPLC, ICP/MS Sniff Out Arsenic in Apple Juice: The Use of Ion Pairing Chromatography with Cation Pairing Reagent Enables Faster Run-Time and Lower Detection Capability during the Analysis of Arsenic in Apple Juice. Chromatography Techniques, p10.
- Heitland P, Köster HD. 2008. Fast Determination of Arsenic Species and Total Arsenic in Urine by HPLC-ICP-MS: Concentration Ranges for Unexposed German Inhabitants and Clinical Case Studies. Journal of Analytical Toxicology 32: 308-314.
- Liquid Chromatography Problem Solving and Troubleshooting. 1994. Journal of Chromatographic Science 32:524 https://doi.org/10.1093/chromsci/32.11.524
Consumables Used
Table 3. Source: PerkinElmer Food Safety and Quality
| Component |
Description |
Part Number |
Reversed Phase
Column |
4.6 mm I.D x 250 mm, 5 μm |
N8145326 |
| HPLC Vials |
HPLC Tested Plastic Vials, 1.5 mL PP |
N9301736 |
| PEEK Tubing |
Yellow, 0.007” ID, 1/16” OD (5 feet) |
N9302678 |
| PEEK Fittings |
Fingertight for 1/16” OD PEEK Tubing |
09920513 |
Nebulizer
Connector |
Column-to-Glass Concentric Nebulizer Connector |
N8152484 |

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Food Safety and Quality.
For more information on this source, please visit PerkinElmer Food Safety and Quality.