The most valuable European agri-food export sector (€10bn in exports, representing a trade surplus close to €9bn) is the spirits drink industry.
Distilled spirits span 46 product categories and are as diverse as the EU’s Member States. The spirits include a variety of geographically specific products which contribute to the culture of their regions and the European Union.
For instance, Scotch Whisky is sold in almost 200 countries worldwide and is the largest category of whisky in the world. Equating to around £ 135 per second to the UK balance of trade, the value of Scotch Whisky exports for 2014 was reported at £ 3.95 billion.
Scotch Whisky and other European spirits are premium global products, and so, they are a major target for illegal counterfeiters.
Counterfeiting has an effect on direct sales, and it damages the reputation of brands and spirit categories. Safety issues are also a worry, and therefore, these effects are relevant to all spirit producers.
To produce fake and adulterated spirits, counterfeiters utilize denatured industrial alcohol, which degrades the product and can pose a significant health risk to those consuming it.
In order to make it undrinkable, there are chemicals that are specifically added to denatured alcohol at trace levels as required by law; if consumed, they can pose a significant health risk.
Some such compounds added to alcohol to prevent consumption are methyl ethyl ketone and methanol. Various analytical methods have been used for detecting adulterated whisky and spirits. Often, these techniques are laboratory based and rely on experienced operators to perform the analysis.
A very simple technique has been developed by utilizing a field-portable GC/MS to detect adulterated Scotch Whiskey and other spirit products. In this article, the analysis of various compounds that can aid in the detection of adulterated Scotch Whiskey will be key.
Other markers in denatured alcohol can easily be detected and positively identified by GC/MS as they are unique in their chemical structure. The mass range of the Torion® T-9 (41-500 m/z) falls just short of being able to detect methanol which is frequently utilized in combination with other compounds as a marker for denatured alcohol.
Yet, the approach outlined here has the ability to reliably detect adulterated spirits without the requirement for detecting methanol and achieving this directly in the field, either at a distribution warehouse, a port of entry or a point of sale, which can help keep these dangerous products from reaching the consumer.
In order to successfully take a sophisticated method like GC/MS to the field, the development of easy-to-use software, robust simple hardware and complimentary sample preparation and introduction techniques was required.
These systems are often used by non-chemists who do not have in-depth training on GC/MS sample preparation and data analysis. The PerkinElmer Torion T-9 is a ruggedized, fully self-contained, field-portable GC/MS.
Battery-operated, with easy-to-use software and an internal carrier gas supply, a non-chemist can easily operate the system in the field.
Successful deployment of the T-9 to the field is also dependent on the development of a sampling technique that is compatible for the task and showing the data analysis of the results to the analyst in a clear, actionable form.
This article will focus on the complete technique development to quickly detect and identify denatured alcohol in Scotch Whisky and other spirits. The technique utilizes the trace compounds added to denatured alcohol in the 1-5 % concentration ranges for detection and identification.
These chemical markers in denatured alcohol possess a unique chemical structure, and GC/MS can easily detect and positively identify them.
The utilization of GC/MS, with its gas chromatography portion as a separation front end, ensures that the flavor and aroma compounds present in spirits do not interfere with the detection by giving rise to false identifications or masking the marker compounds.
The high sensitivity of the GC/MS technique ensures positive marker detection, even at very low concentrations, as counterfeiters might try to chemically remove some of the markers, further lowering the concentrations to prevent detection.
Method Development
Based on previous experience, a general purpose GC/MS technique was chosen for the analysis. Table 1 lists the method parameters.
Table 1. Method Parameters. Source: PerkinElmer Food Safety and Quality
| SPME |
PDMS/DVB |
| GC Injector |
270 °C |
| GC Column |
MXT-5 5 m x 0.1 mm id, 0.4 um df |
| GC Carrier Gas |
50 °C initial hold for 10s, programmed at 2 °C/s to 270 °C |
| GC Column Temp. |
43.92 |
| GC Run Time |
2 minutes |
| Injection |
Split 10:1 |
| Mass Analyzer |
Toroidal ion trap |
| Scan Range |
41-500 |
| Transfer Line |
250 °C |
| Ionization |
-70 eV in trap electron impact |
Next, standards of the denatured marker compounds at concentrations in the mid-ppm and low-percent range were spiked into a mixture of water with 40 % ethanol.
Initially, samples were prepared by pipetting 1 ml of sample into a 10 ml headspace vial, which was then sealed with Teflon coated septum. The samples were left to equilibrate for 10 minutes.
The volatile compounds in the mixture will partition between the liquid and the headspace above the liquid in the sealed container. In order to determine an optimum sampling time, the headspace above the liquid was sampled for periods of time ranging from 30 seconds to five minutes.
Some steps in the optimization of the technique are the experimentation with the various sampling times and concentration ranges. To sample the headspace of the sample container, the Custodion™ SPME sampling syringe was employed. The SPME fiber is coated with an adsorbent material and is 1 cm in length.
A wide-boiling-point range, general-purpose, fiber coating was chosen for this application (PDMS): polydimethylsiloxane/ (DVB) divinylbenzene. Volatile components are collected on the fiber for subsequent injection into the GC/MS by exposing the fiber to the headspace above the sample.
The prepared standards were used to build a reference library (target compound method) of denatured marker compounds.
The target compound library is made up of MS mass spectra and the GC retention time; these two parameters are employed for orthogonal identification, meaning that two different sources of information are employed at the same time to ensure identification accuracy.
A list of the reference library for marker compounds, which can be found in American, German and European denatured alcohol products, are detailed in Table 2.
Table 2. Target List. Source: PerkinElmer Food Safety and Quality
| Compound |
Retention Time |
CAS Number |
Mass Spectrum |
| Isopropyl Alcohol |
11.30 |
67-63-0 |
44, 45*, 47,59,61,69 |
| Methyl ethyl ketone |
16.88 |
78-93-3 |
43, 57, 72, 73* |
| Methyl isopropyl ketone |
21.99 |
563-80-4 |
43*, 71, 86, 89 |
| Methyl isobutyl ketone |
29.42 |
591-78-6 |
43, 57, 58, 85,100,101* |
| Ethyl sec-amyl ketone |
48.78 |
541-85-5 |
43, 57, 71, 72, 73, 99,128,129* |
*Represents base peak
The technique was tested for detection of the denaturants by spiking European and German method denatured alcohol into a blended Scotch Whisky. Both produced positive detection for the denatured compounds when analyzed.
GC/MS results are usually shown as a list of chemical compounds which have been detected. An analyst knows how to interpret the data in the laboratory, but in the field, the non-specialist will not necessarily know that the detection of methyl isopropyl ketone represents the detection of denatured alcohol.
Figure 2 is an example of the alert screen which is shown on the T-9 when one or more compounds in the target library are detected.
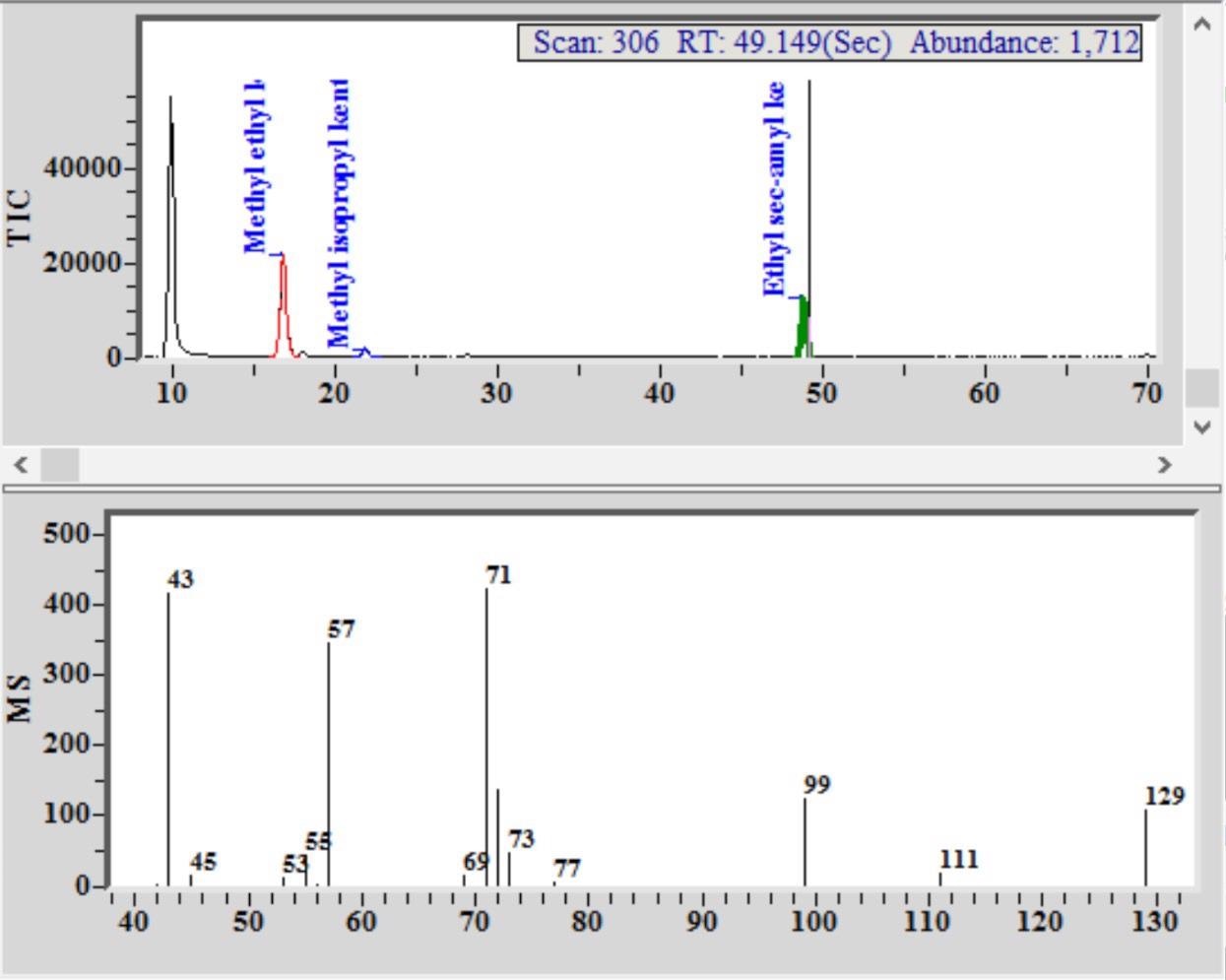
Figure 1. Chromatogram and mass spectrum, with identification of marker components in a Euro spike of a blended Scotch Whisky. Image Credit: PerkinElmer Food Safety and Quality
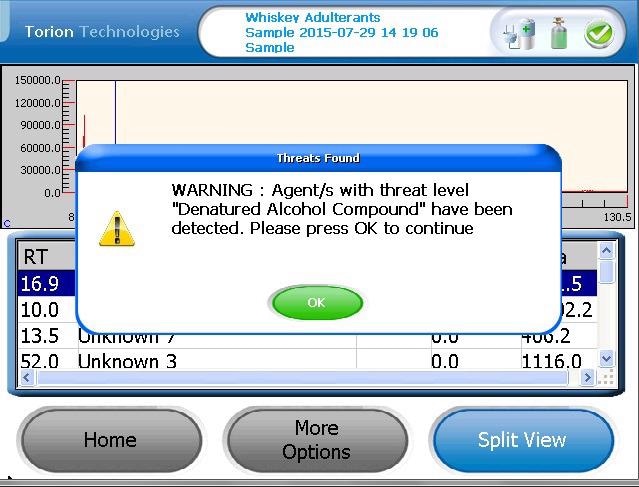
Figure 2. An example of an ‘Alert’ when a target compound in denatured alcohol is detected. Image Credit: PerkinElmer Food Safety and Quality
The T-9 software includes the ability to flag specific compounds as hazardous, and the use of hazard levels alerts the operator that one or more of the denatured alcohol markers has been detected.
Method Validation
Tests were then carried out to establish the minimum level of identification, and dynamic range of identification, by utilizing one of the blended Scotch Whisky products.
Denatured alcohol from the USA was spiked into the whisky at levels ranging from .1 % (1000 ppm/v) to 10 % (100,000 ppm).
The Material Safety Data Sheet (MSDS) for the denatured alcohol product lists the component concentration ranges as 1 – 4 % for (MIBK) Methyl Isobutyl Ketone in the product.
When using a spike of 0.1 to 10 % denatured alcohol, the resultant concentration range for the MIBK is approximately 10 – 4000 ppm/v. The lower level of identification was established as 0.25 % denatured alcohol or from 25 to 100 ppm/v of MIBK (1- 4 % concentration in the denatured alcohol).
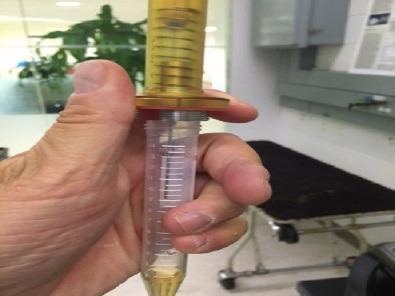
Figure 3. Sampling with the CUSTODION. The SPME fibre is housed in a durable syringe that is operated like a ball point pen. Image Credit: PerkinElmer Food Safety and Quality
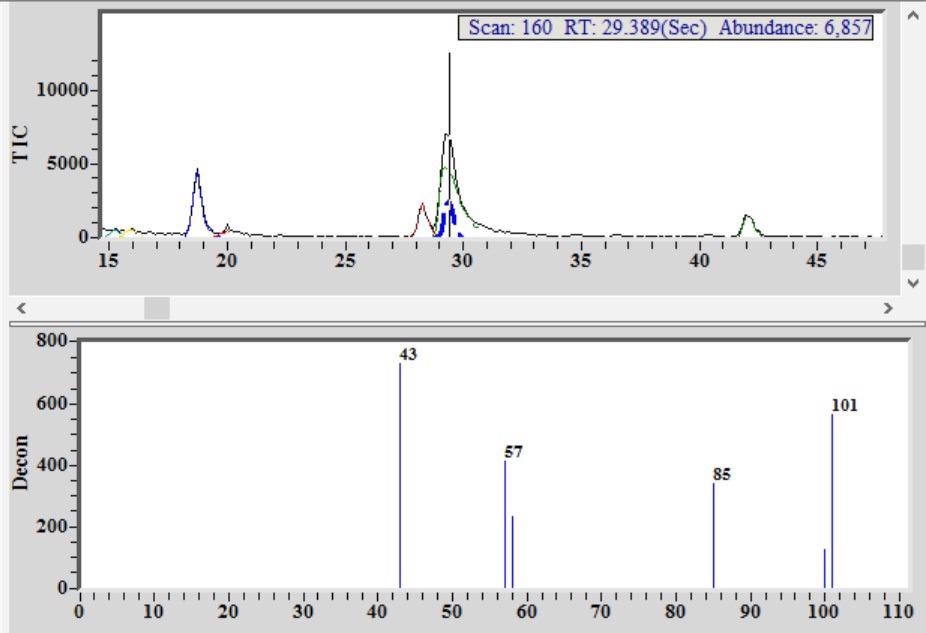
Figure 4. Chromatogram from single malt Scotch whiskey showing detection and identification of methyl isobutyl ketone (MIBK) co-eluting with a flavor/aroma compound. Image Credit: PerkinElmer Food Safety and Quality
A simplified sample preparation step was developed during this next stage of method validation. The concept was to make sampling as cost-effective and as easy as possible in the field.
The utilization of the 10 ml headspace vials employed during initial technique development would be cumbersome and costly in the field.
Frequently utilized in laboratories, polypropylene autosampler tubes (15 ml), provided an easy-to-use, cost-effective field sampling device (PerkinElmer part # B0193233).
Table 3. The spirit drinks employed in this study. Source: PerkinElmer Food Safety and Quality
| Qty |
Spirit Drinks |
| 2 |
Blended Scotch |
| 2 |
Single Malt |
| 2 |
Tennessee whiskey |
| 1 |
Canadian whiskey |
| 1 |
Irish whiskey |
| 3 |
Rums (2 flavoured) |
| 2 |
Vodka (1 flavoured) |
| 1 |
Tequila |
| 1 |
Liquor |
So the spirit drink can be easily poured into the tube, the tubes are graduated to ensure an accurate volume. The utilization of the sampling tubes eliminated the requirement for pipetting and the use of a crimper to seal the vial. This, therefore, decreases the cost of consumables required for the analysis.
The sample preparation steps are:
- Adding 2 ml of the spirit to be tested to the sampling tube
- Capping with the screw cap top provided
- Equilibrating for five minutes
The cap is removed after five minutes, and the CUSTODION SPME syringe is inserted into the headspace and held firmly against the top of the tube. The SPME fiber is then exposed to the headspace for 30 seconds. Next, the sample, adsorbed onto the SPME fiber, is then injected into the GC/MS for analysis.
This method was used to prepare the 15 spirit drinks. They were sampled and run unadulterated with zero false positive detection events. Denatured alcohol was then spiked into the drinks at a concentration of 0.25 %, and all 15 samples were then run with 100 % positive detection.
The spirits were then spiked with a denatured alcohol concentration of 10% and again, the denatured marker MIBK was positively detected.
Further simplifying the reporting so that only compounds listed in the target library are reported was the final step in method development.
Summary
The utilization of field-portable GC/MS supplies fast, reliable determination of the presence of denatured alcohol when added to spirit drinks to raise the alcohol content.
Based on the addition of 0.25 % by volume of denatured alcohol, the low detection limits ensure confidence that, even if steps are taken to partially remove the denatured alcohol markers, they will still be detected.
It should also be noted that the test presents a more challenging scenario than would be expected in practice: that is, the utilization of 100 % denatured alcohol as a counterfeit white spirit.
The analysis time is around 10 minutes per sample. So that there is no ambiguity for the operator, the data analysis software has been set up to give results in a “non-chemist” format.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer Food Safety and Quality.
For more information on this source, please visit PerkinElmer Food Safety and Quality.