Cathodoluminescence (CL) microscopy is a highly effective and respected microanalysis tool in geosciences. This tool works by analyzing the light emitted by a mineral or gemstone, which is excited by an electron source.
This technique is a popular choice where there is a need to determine mineral provenance as part of geochronology and metamorphic alteration studies. More recently, this technique has also begun to see regular use in thermobaromtery for petrographic applications.
Crooks first reported the phenomenon of cathodoluminescence in 1879 after examining the blue-green light emitted from synthetic calcium sulfide crystals bombarded with a cathode ray.
In their initial publication, Crooks had reported that “many natural minerals and gems shine with great splendor when struck by a high energy electron beam,” highlighting that “[d]iamonds from other localities shine with different colors, such as bright blue, pale blue, apricot, red, yellowish-green, orange, and bright green.”
This report provides some initial insight into why CL is a vital microanalysis tool. The light emission mechanism of cathodoluminescence may represent a complex process in minerals and gems. These insulator materials are typically characterized by an electronic structure with a wide forbidden energy gap.
Despite this, the observed luminescence is typically directly linked to trace element impurities or crystal defects, with recent developments in CL detector sensitivity enabling microanalysis at the parts per million level.
Photons emitted throughout the CL process are generally described by their polarization, wavelength and angular distributions (Figure 1). However, practical geoscience applications tend to regard the photon emission rate and wavelength distribution (color) as the photons’ most essential properties.
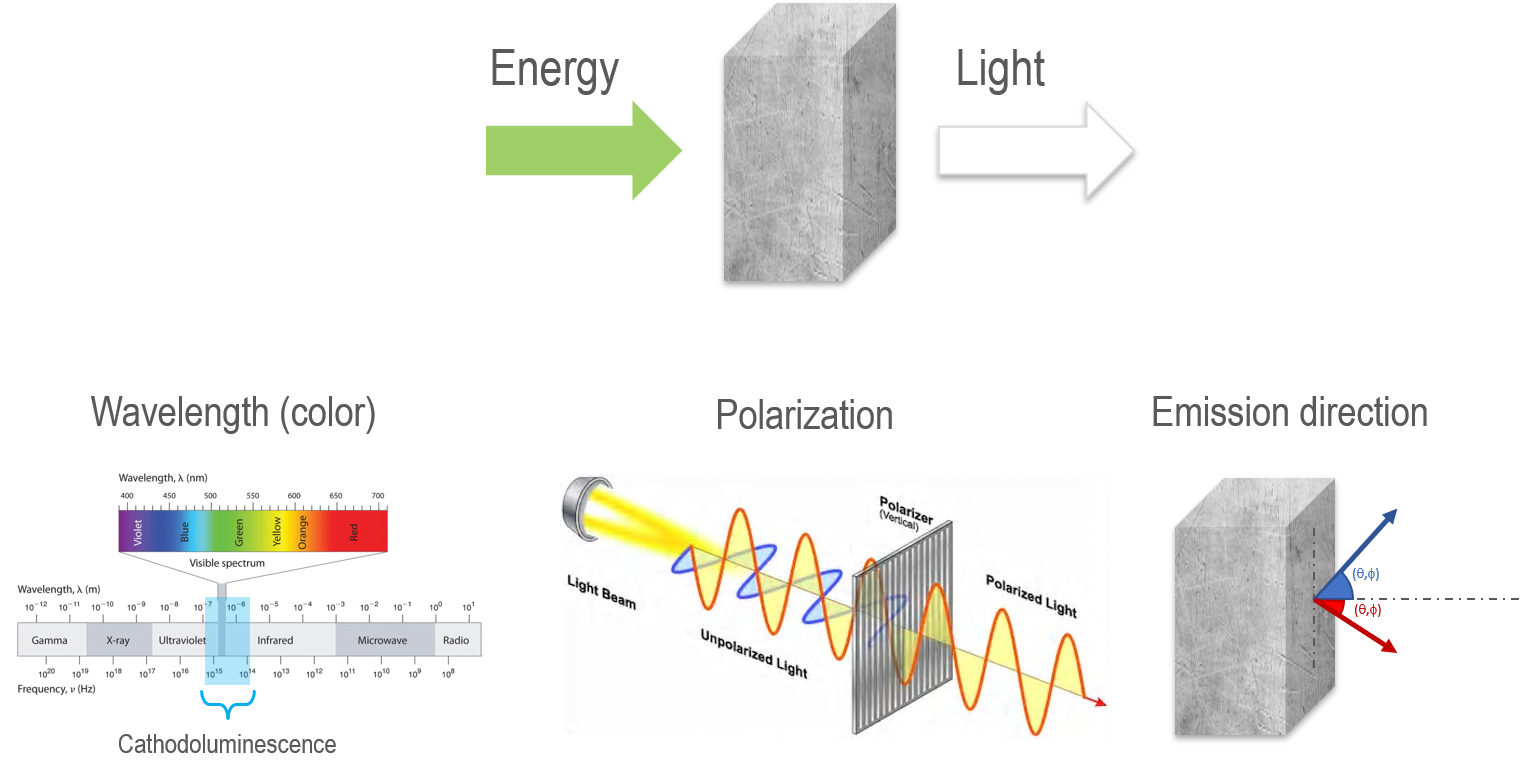
Figure 1. Photons emitted in the CL process. Image Credit: Gatan Inc.
CL’s use became more widespread in the 1960s and 1970s, which saw CL images acquired via an optical microscope and an electron flood gun attachment.
These images were employed in the reconstruction of mineral formation processes, as well as being used to document alteration and the growth textures not observable via other techniques.
Figure 2 displays examples of cross-polarization, backscattered electron and cathodoluminescence images acquired from the same (partially decomposed) zircon. Growth textures only observable in the CL image are also displayed.
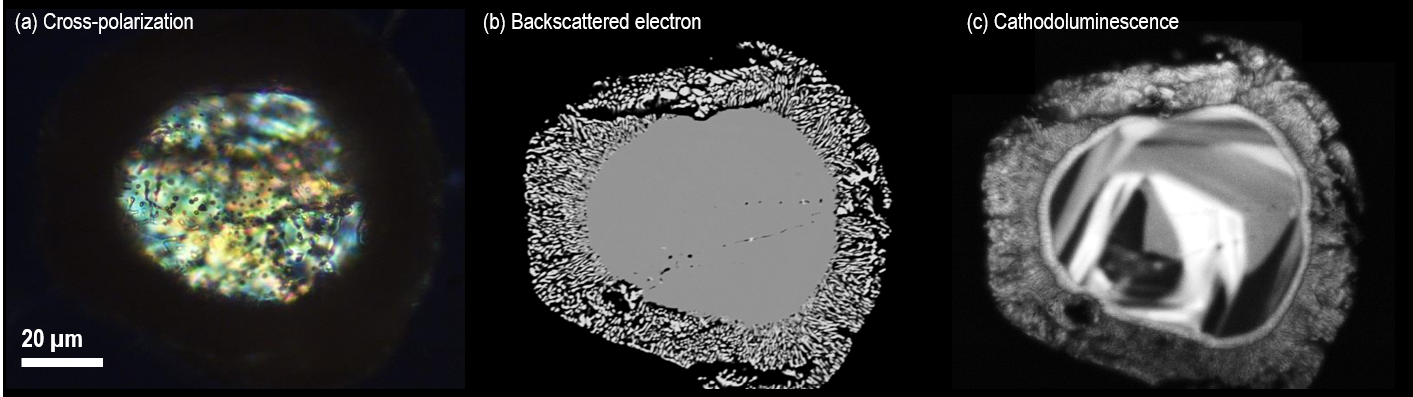
Figure 2. Images of the same zircon grain acquired using different analysis techniques; the zircon is a partially decomposed zircon in Mistatin Lake impact melt glass. The cross-polarized light microscope image (a) reveals a core and rim structure with some inhomogeneity in the central region, the backscattered electron image (b) captured using an SEM reveals a decomposed rim and core and, the cathodoluminescence image (c) reveals internal texture not observable by the other imaging techniques. Images courtesy of Michael Zanetti, Washington University in St. Louis and the McDonnell Center for the Space Sciences, Earth and Planetary Science Dept. Image Credit: Gatan Inc.
There have been significant developments in electron microscopy and CL detectors over the past 50 years. It is now possible to acquire CL maps from entire 1” x 2” thin section samples and with spatial resolutions approaching the nanoscale, thanks to the move to CL in the SEM.
A diverse array of CL detectors for the SEM is commercially available, offering a wide range of analytical capabilities.
This article looks at the different operating modes of contemporary CL detectors that can be applied to routine geological applications, with a view to streamlining and assisting the process of choosing a CL detector.
CL Detection Modes in an SEM and their Application in the Geosciences
CL detection involves a focused beam of electrons impinging the sample under investigation. This process induces light emission in the electromagnetic spectrum’s near-infrared, visible and ultraviolet wavelength ranges.
An optical detection system is then used to collect and analyze this emitted light. The SEM’s electron beam can be placed with nanometer precision. If required, this can be scanned across the sample’s surface in a stepwise manner to facilitate CL emission analysis at each individual step.
Spatial Information: Unfiltered Imaging
Unfiltered imaging is occasionally referred to as polychromatic, panchromatic or integrated intensity imaging.
The photon emission rate’s spatial distribution is recorded without the possibility of dispersion or filtering this data by wavelength. Data is shown as black and white images. The intensity grayscale in these images corresponds to the measured photon rate (Figure 3).
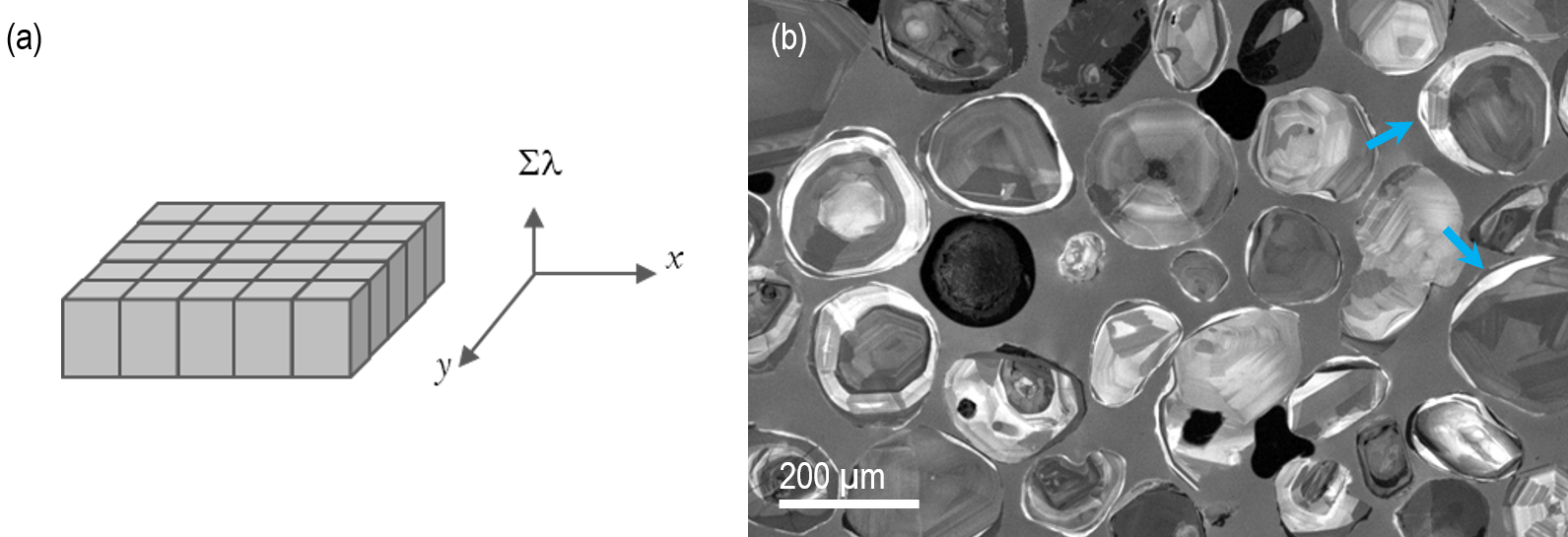
Figure 3. (a) Schematic representation of an unfiltered cathodoluminescence image—the integrated photon flux is plotted pixel-by-pixel across two spatial dimensions. (b) An unfiltered CL image of a collection of polished zircon grains revealing internal texture related to (re-)crystallization. Some zircons exhibit late rims (indicated by arrows) where the grain exteriors date to a later geological period than the core. Images captured using the MiniCL™ detector. Image Credit: Gatan Inc.
A CL detector can be as rudimentary as a photon-sensitive device located close to the sample’ for example, a photomultiplier tube (PMT) or silicon diode. Depending on the detection equipment’s sensitivity, unfiltered images may be captured in a few seconds or tens of seconds. This simple form of CL mapping provides all necessary information for a limited number of applications.
CL maps are used in geochronological investigations to help visualize late rim formation in zircons during U/Pb radiometric dating.
These relatively routine studies see zircons used as a geological ‘clock’ due to the crystal structure’s incorporation of small amounts of radioactive U isotopes and their decay products during crystallization.
Where zircons have experienced multiple crystallization events – for example, zircons with (old) inherited zircon cores found in younger igneous zircons – the U/Pb isotope ratio in each crystallization period will vary depending on the time elapsed. In this context, U is immobile in the zircon crystal.
It is not always possible to recognize this polyphase structure using conventional imaging methods like light microscopy, but this remains necessary in ensuring accurate dating results.
The application of CL in the SEM will enable zircons to be selected for dating purposes.
Zircon phases of varying ages can be identified by examining the late rims, which appear as bright regions at the grain perimeter of an unfiltered CL image. These images also allow conclusions to be drawn regarding the origin and development of the inherited phases in comparison with detrital zircons.
Spectral Information: (Single Point) Cl Spectrum
The emitted light’s wavelength distribution is recorded from the region or point of the sample that is exposed to the SEM’s electron beam. The resulting data is displayed by plotting intensity against wavelength or energy.
During this process, wavelength and energy can be considered to be inversely related.
A spectroscopic CL detector generally features a light-collecting objective that works in conjunction with an optical spectrometer; for example, a spectrograph or scanning spectrometer (with PMT).
When using a spectrograph, it is possible to capture a wavelength-resolved spectrum in a single acquisition using a pixelated detector such as a charge-coupled device (CCD).
The CL spectrum may also be captured by scanning a range of wavelengths and recording the light intensity at each scanned wavelength. This is known as a wavelength-filtered spectrum.
In terms of acquisition time, it is possible to capture a wavelength-resolved spectrum in a few milliseconds to a few seconds, while a wavelength-filtered spectrum generally requires several tens of seconds to acquire.
Application
CL spectroscopy can be employed in microanalysis. In this context, CL is able to reveal the presence (or absence) of the trace element or defect centers associated with a CL image’s features.
For example, luminescence in calcite (CaCO3) is linked to the presence of Mn2+, Pb2+, Ce3+, Fe3+, Sm3+ and Dy3+ trace elements, with each trace element resulting in the sample exhibiting luminescence at a different wavelength.
Cr3+, Fe3+ and Mn2+ luminescence centers have also been measured in Beryl (BeAl2Si6O18) (Figure 4).
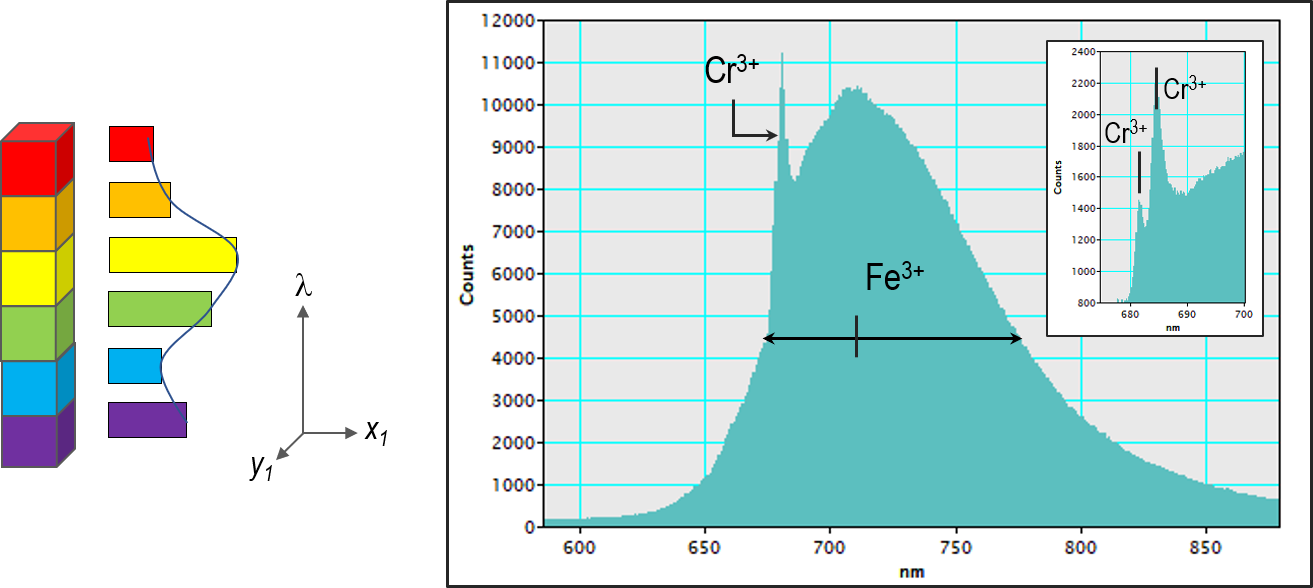
Figure 4. Cathodoluminescence spectrum from a single point or region of a sample. (a) schematic representation and b) CL spectrum captured from a beryl crystal with luminescence peaks associated with Cr3+ (peaks at 681.24 and 683.6 nm with FWHMs 0.88 and 1.53 nm respectively) and Fe3+ (720 nm with FWHM 110 nm).Spectrum captured using the Monarc™ detector. Image Credit: Gatan Inc.
The SEM’s electron beam places the sample into an energetic state through the generation of free carriers – both electrons and holes. CL is one notable mechanism that sees the sample return to the ground (energy) state via the release of photons.
Many different factors impact CL’s intensity and wavelength, including:
- The mineral’s band-gap energy
- The presence of structural defects such as vacancies or dislocations
- The concentration of any trace elements present
These latter centers may introduce (mid-gap) energy states or allow intra-ion energy transitions; for example, those exhibited within the f orbitals of rare earth elements (REE) or the d orbitals of transition metals.
Electronic transitions taking place between these mid-gap or intra-ion states can release photons with wavelengths that directly correspond to the change in energy. Wavelength discrimination (in the form of CL spectroscopy) therefore facilitates the detection or differentiation of trace elements.
Concentrations of elements needed to produce measurable peaks in a CL spectrum may be orders of magnitude lower than those measurable by X-ray microanalysis – whether this is performed in energy-dispersive (EDX) or wavelength-dispersive (WDX) mode.
Spatial and Spectral Information
Some CL detectors are able to collect both spatial and spectral information within one data set.
Spatial information typically takes the form of images or maps, providing useful insight by contrasting different phases, visualizing defects and zoning the internal structures of solids.
Spectral information, on the other hand, facilitates the detection of trace elements and the trace elements’ valence and structural position. The terminology used to describe this spectral data typically depends on the specific spectral information contained in the dataset:
Wavelength-Filtered Imaging
Spatial information in a wavelength-filtered image is only captured via a defined wavelength or an extremely limited range of wavelengths. Wavelength-filtering is generally performed by detecting light passing through the wavelength selecting (exit) slit of an optical transmission filter or a scanning spectrometer.
An optical spectrometer offers the most flexibility in the selection of the wavelength and wavelength range (bandpass), but images generated in this manner tend to feature poorer signal-to-noise ratios versus equivalent images captured using an optical filter.
This difference is primarily due to losses at each optical surface through the spectrometer.
Scanning spectrometers are widely used despite this limitation because it is impractical to maintain hundreds (or even thousands) of optical filters with the desired transmission parameters.
Wavelength-filtered images allow mapping of the spatial distribution of an emission center. It is also possible to capture images of multiple emission centers via repeated scans of the electron beam.
The filtered wavelength is changed for each scan under these circumstances. Emission centers with well-separated emissions can be mapped using this approach.
Figure 5 shows the spatial distribution of Dy3+ and Er3+ rare-earth ions in a melt glass zircon. These have been mapped using wavelength-filtered imaging at 575 and 400 nm, respectively.
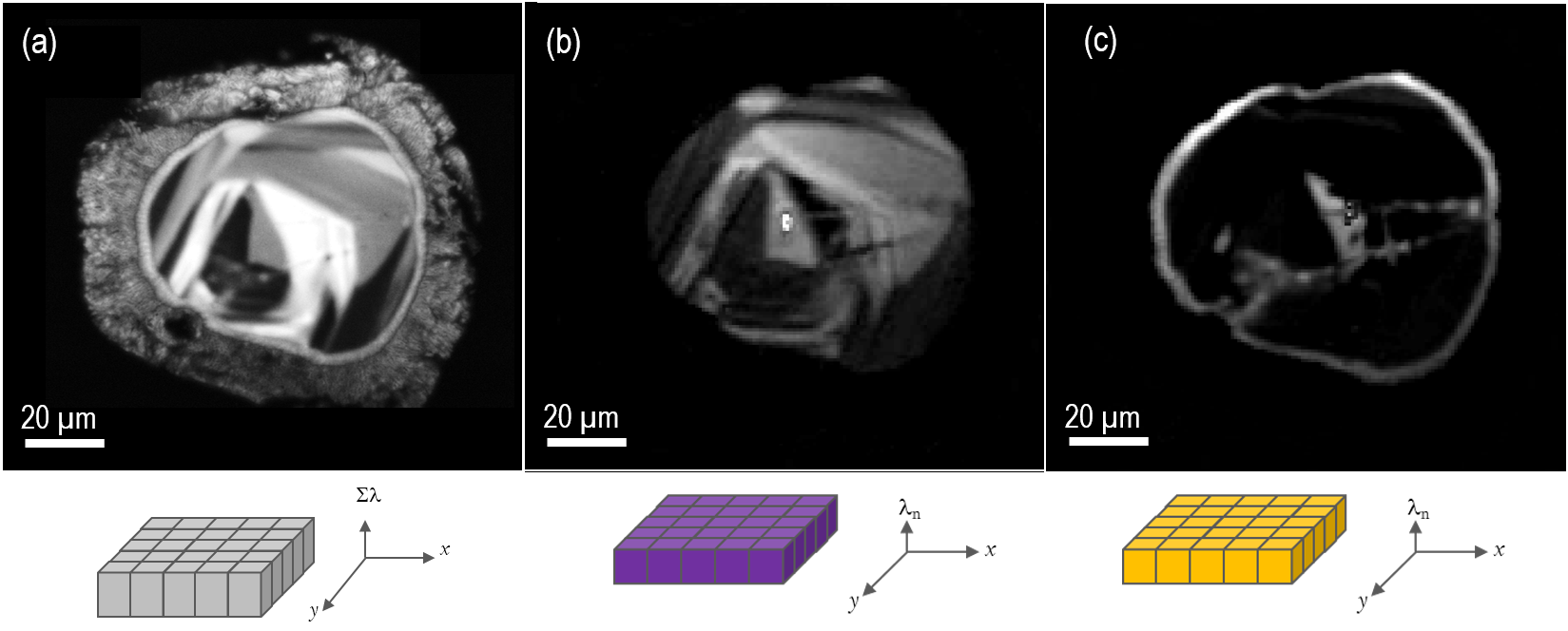
Figure 5. CL images of the same zircon as shown in Figure 2. Image a) unfiltered CL image revealing internal texture, b) and c) wavelength-filtered CL images at 404 and 575 nm respectively corresponding to Er3+ and Dy3+ trace rare-earth ions; the wavelength-filtered images have been processed by removal of the ZrSiO4 background signal at 650 nm. The Er3+ and Dy3+ concentrations were in the range 10 – 300 ppm, as determined by microanalysis in the electron probe microanalyzer. Schematic representations of the acquisition modes used are displayed immediately below the respective CL image. Images captured using the MonoCL4™ Elite detector and provided courtesy of Michael Zanetti, Washington University in St. Louis and the McDonnell Center for the Space Sciences, Earth and Planetary Science Dept. Image Credit: Gatan Inc.
It is typically possible to capture wavelength-filtered images in a few tens or hundreds of seconds.
Color Imaging
Color imaging sees spatial information captured at three wavelengths which correspond to the visible spectrum’s red, green and blue components. These components are then recombined to form a color image in a similar fashion to the screen of a color television.
Specialized detectors – for example, the ChromaCL2™ detector – are able to simultaneously capture the color components, enabling color image capture during just one pass of the electron beam.
Sequential scans of the electron beam are more commonly required, however, with color components contributing to the signal change between scans.
It is generally possible to capture color images in a few tens or hundreds of seconds.
Application
Spectrally resolved CL imaging has seen considerable use in metamorphic petrology applications, for example, identifying mineral distributions and uncovering primary and secondary growth features such as recrystallization, fluid flow, deformation, alteration and mineral neoformation.
Access to this additional spectral information supports the accurate interpretation of CL data. Figure 6a provides an example unfiltered CL image of plutonic quartz, revealing regions with lower CL intensity in the grain interiors and healed fractures.
These two regions initially seem equivalent due to their identical grayscale intensity, but the application of color imaging reveals very different CL emissions. The regions adjacent to healed fractures in this example exhibit red colors, indicating recrystallization at low temperatures (Figure 6b).
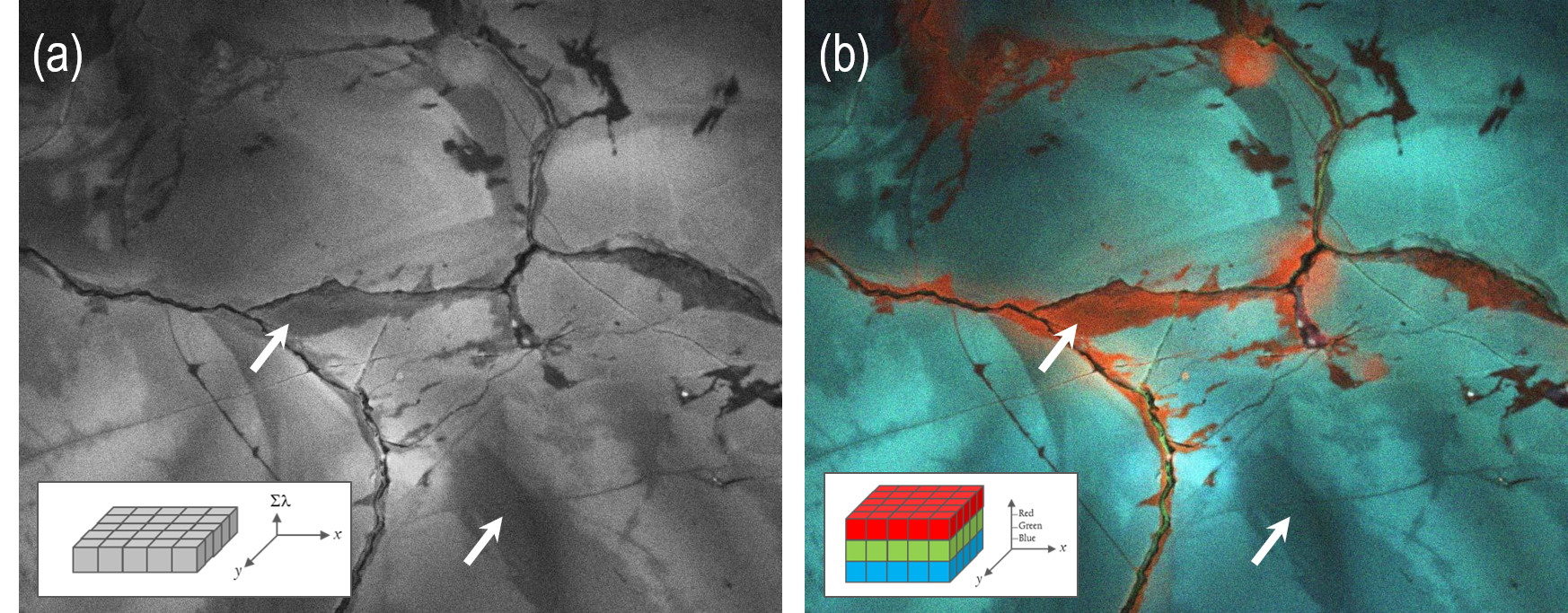
Figure 6. a) Unfiltered and b) color cathodoluminescence images of the same region of igneous quartz (plutonic granite); inset schematics represent the mode of data acquisition with the color image formed using three spectral components corresponding to red, green and blue wavelengths). The arrows indicate two regions of the mineral that, in an unfiltered CL image, appear equivalent. However, the additional spectral information available in the color image reveals that the two regions are distinctly different, reducing the risk of incorrect interpretation. Images captured by ChromaCL2 detector and provided courtesy of Dr. Juergen Schieber, University of Illinois. Image Credit: Gatan Inc.
CL imaging has also been used widely in the analysis of quartz, particularly in cases where detrital and authigenic quartz is readily discernible. Color CL imaging has facilitated the assessment of metamorphic grade in cases where inherited CL signatures are noted to fade with increasing metamorphic grade.
For instance, detrital blue CL colors tend to fade with increasing temperature. These become overprinted by red tones until fresh metamorphic blue CL appears at Staurolite grade at the margin of grains, prompting the detrital signature to be lost (Figure 7).
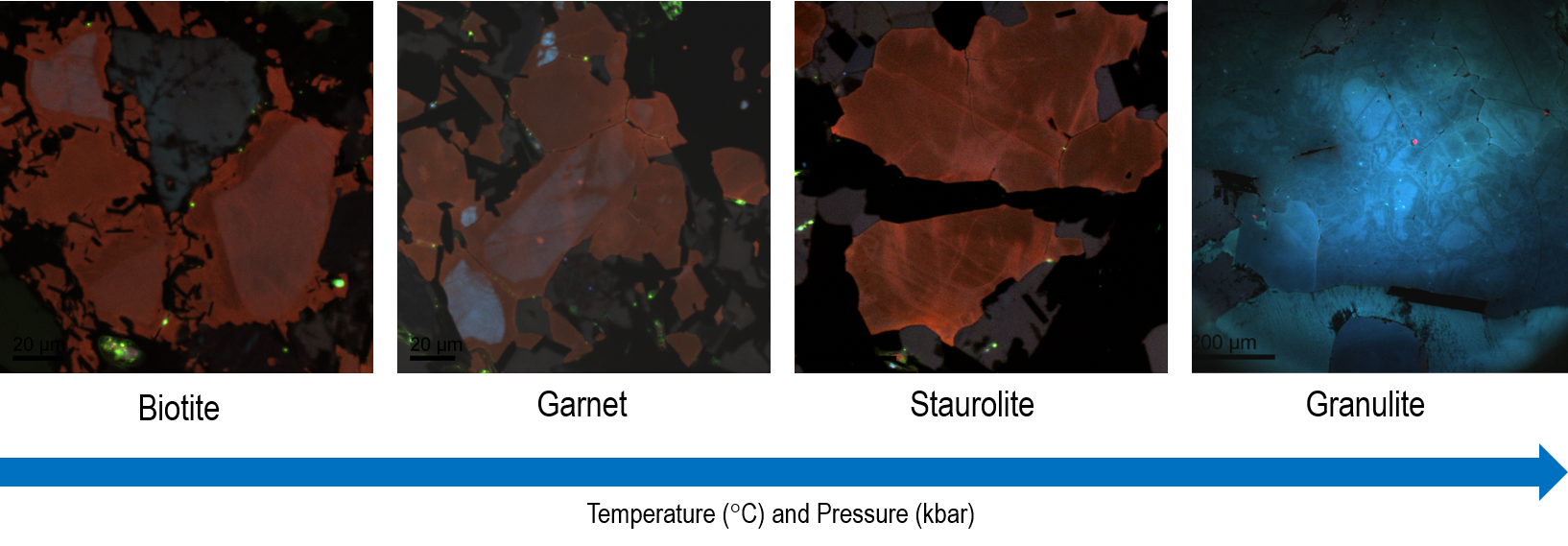
Figure 7. Color cathodoluminescence images of metamorphic quartz samples from the Buchan Metamorphic Sequence, Scotland. Color CL images reveal textural differences associated with the grade of metamorphism with the fading of detrital blue colors and inherited texture with increasing temperatures and pressures until fresh metamorphic CL features appear at Staurolite grade at the margin of grains. Images captured by ChromaCL2 detector and provided courtesy of Dr. Juergen Schieber, University of Illinois. Image Credit: Gatan Inc.
This feature has recently allowed color CL to infer the provenance of shales and mudstones using even silt-sized grain populations.
Within this specific application, the visible colors and textures facilitate an assessment of whether a population of quartz grains is volcanic, metamorphic or plutonic derived. It is even possible to gain insight into the intensity of metamorphism.
Provenance assessments of shales have historically utilized bulk rock geochemical parameters, but these are prone to bias by diagenesis or weathering. Provenance determination using SEM Color CL employs highly resistant grains and is much more rapid, representing an overall superior methodology.
Spectrum Imaging
Spectrum imaging is also known as hyperspectral imaging or spectrum mapping. A ‘spectrum’ or ‘hyperspectral’ image is a dataset that includes both spatially- and spectrally-resolved information.
This spectrum can essentially be understood as a two-dimensional array of spectra or as an aligned stack of wavelength-filtered images. Figure 8 provides a conceptual representation of this data cube.
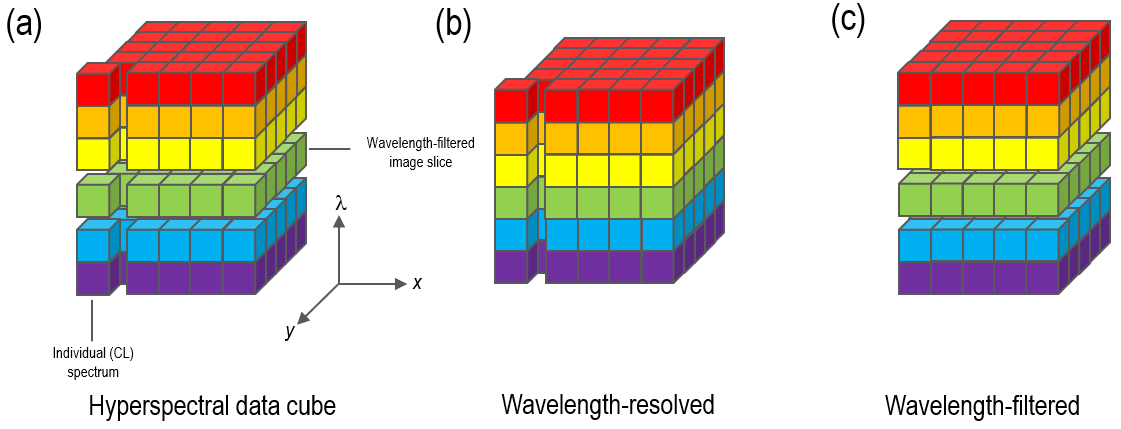
Figure 8. Schematic representation of a hyperspectral image data cube a) showing the stack of wavelength-filtered images as horizontal slices and the component spectra as vertical columns; b) wavelength-resolved acquisition mode where an array detector is used to capture complete CL spectra on a point-by-point basis; c) wavelength-filtered acquisition mode where a photomultiplier detector is used to capture an aligned stack of wavelength-filtered images. Image Credit: Gatan Inc.
The term ‘hyperspectral image’ was initially used to describe a data cube that featured a large number of wavelength bands (typically >20). This is different from color imaging or multiple wavelength-filtered imaging, which see spectral information limited to one or a few wavelength bands.
A number of electron microscopy and spectroscopy signals can be employed in spectrum imaging, such as X-ray and electron energy loss spectroscopies.
CL spectrum imaging was first introduced in the 1990s and used in the analysis of optoelectronic semiconducting materials.
Since that time, it has seen increased use used in this field due to its ability to map shifts in emission peak wavelength resulting from spatial variations in properties such as alloy composition, elastic strain, electric field or temperature.
This specific feature is less beneficial in the analysis of minerals, but it does offer several advantages which have led to its increasing use in geoscience.
Advantages of Hyperspectral Imaging
The clearest advantage of measuring CL in hyperspectral mode is that the full range of spatial and spectral information is captured in a single measurement - there is no need to have prior knowledge of the sample.
While a spectrum image could be considered nothing more than many discrete wavelength-filtered maps of the sample, in some instances, there may be small inclusions within the mineral that only luminesce at a particular wavelength.
These features would likely be missed by both wavelength-filtered imaging and (point) spectroscopy, while the spectrum imaging approach would capture this information. This would allow these features to be revealed in later data analysis.
Another notable advantage of the spectrum imaging mode is its capacity to deconvolve spectral features, allowing the intensity of overlapping peaks to be mapped or for peaks to be separated from backgrounds.
This type of deconvolution can be achieved via the application of peak fitting or multivariate statistical analysis methods.
While the advantages of the spectrum imaging approach are clear and it is already widely used in material science and nanophotonic studies, there has thus far been only a modest uptake of this technique in geoscience research.
This has partly been driven by the historically high cost of commercial CL detectors with spectral imaging capabilities. More notably, it has also been caused by the necessary reduction in mapping speed, resulting in maps with modest spatial sampling being highly impractical to acquire.
For example, maps formed with 256 x 256 pixels – containing 65,536 pixels in total – sampled at a rate of 1 spectrum per second would require 18 hours to collect. Thankfully, the release of the Monarc™ detector is expected to change and improve these practices in geoscience.
Many commercial CL systems feature optical designs which place stringent requirements on the co-alignment of the sample, CL system and the optic axis of the SEM. If all components are not aligned correctly, this can lead to >98% light losses.
The manual nature of the multi-part alignment process has historically been highly challenging, but the Monarc detector has been specifically designed to address this severe limitation of all previous spectroscopic-CL systems for the SEM.
The Monarc detector boasts advanced auto-alignment methods which have been developed to ensure ideal alignment of all three components, irrespective of the sample type and the user’s level of expertise.
Software algorithms are able to identify and track the electron beam scan pattern’s center, using this to align the CL collection mirror and bring the specimen to the CL system’s ideal focal point.
This method of alignment guarantees optimal collection conditions, significantly lowering the acquisition time per spectrum while avoiding optical losses commonly associated with misalignments.
While it appears to be a relatively simple upgrade, this advance makes spectrum imaging a practical and viable acquisition method for virtually any sample and all users. Using the Monarc system, even low luminescent samples such as quartz can be routinely analyzed in tens of minutes instead of tens of hours.
Spectrum image data acquisition is traditionally collected using an array detector, on a pixel-by-pixel basis, a single (wavelength-resolved) spectrum at a time. Array detectors are able to capture 1024 – 2048 wavelengths (colors) in parallel while offering high spectral resolutions (0.1 – 1 nm), but these are limited to acquisition rates ~5 ms per pixel due to limits in array detectors’ readout speeds.
A wide range of rocks and minerals feature spectra with natural line widths 10 – 150 nm full width half maximum, however – far wider than most instruments’ available spectral resolution.
The Monarc detector is able to collect spectrum images via an array detector, but this instrument also offers an alternative approach to collecting spectrum images with much higher acquisition rates and lower spectral resolution.
This rapid method can still accommodate the requirement for many minerals, but instead of filling the spatial-spectral data cube with wavelength-resolved spectra on a point-by-point basis, a series of (aligned) wavelength-filtered maps is collected and referred to.
This acquisition method is known as ‘wavelength-filtered spectrum imaging.’
This acquisition mode sees a PMT detector employed in the collection of n wavelength-filtered CL images. The pixel time in each image slice can therefore be collected thousands of times faster than is possible with an array detector.
This approach also allows spectrum images with 167 wavelength channels to be collected up to 30 times faster than the wavelength-resolved approach.
The number of sampled wavelength channels is user-defined. The example presented in Figure 9 is a polished zircon grain that required 42 wavelength channels to resolve almost all spectral features, but with >70x higher spatial sampling thanks to the method used.
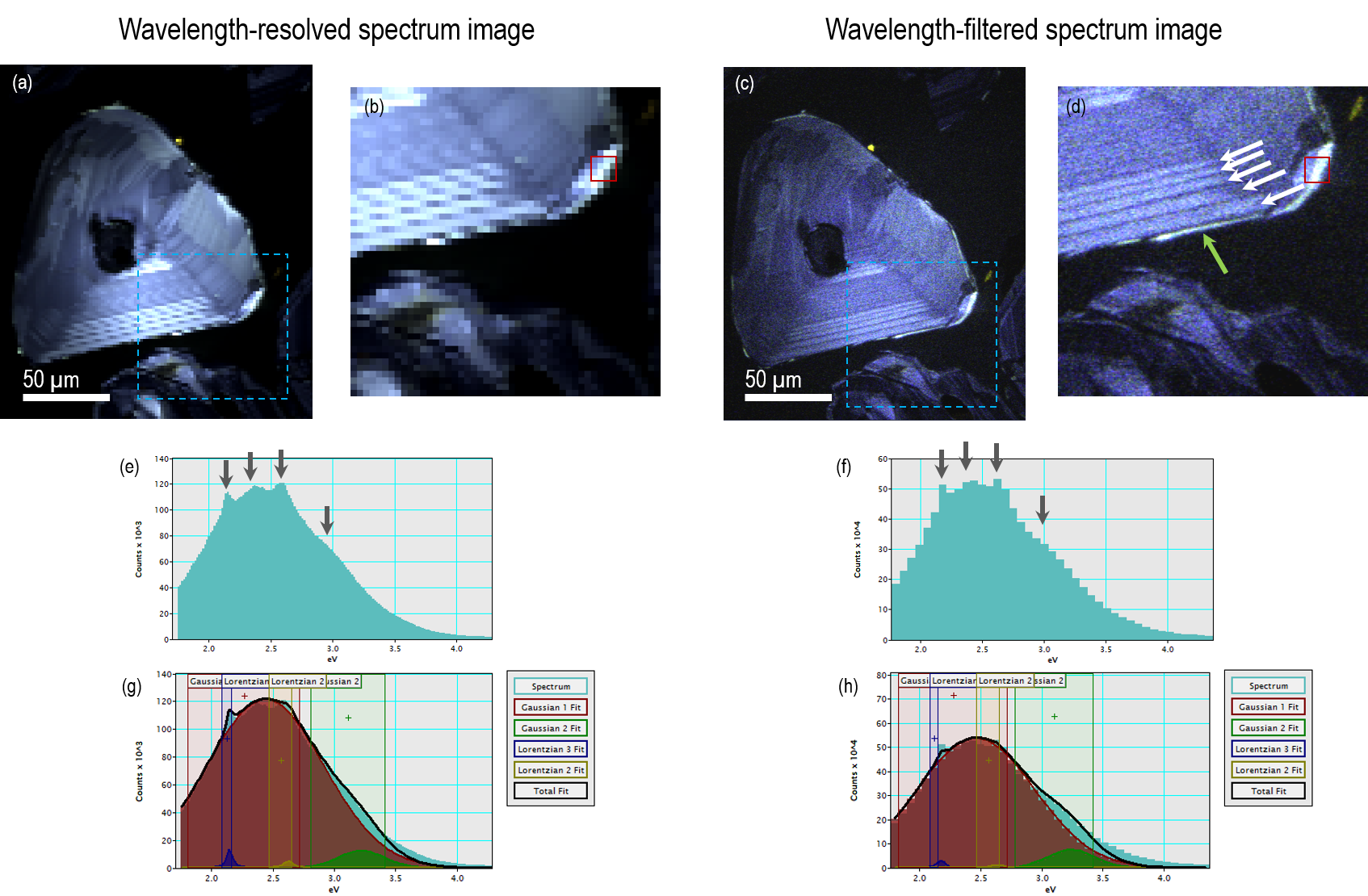
Figure 9. a) – d) True-color representation of two hyperspectral CL data cubes of the same polished zircon grain. Images a) and b) were captured using the wavelength-resolved spectrum imaging mode, schematically represented in Figure 8b and c) and d) were captured using the wavelength-filtered spectrum-imaging mode represented schematically in Figure 8c. Images b) and d) are close up of the region contained within the blue dotted square on images a) and b), respectively. Images e) and f) are two spectra extracted from the regions of the spectrum images indicated by the red rectangle in c) and d). The same spectral features are identified in both data sets and can be described by four peaks (two gaussian and two Lorentzian) with the peaks at 2.15 and 2.51 eV corresponding to Dy3+ rare-earth ion. The two hyperspectral data cubes were captured in 150 s using the Monarc detector. Image Credit: Gatan Inc.
It has also been shown that the quantitative thermobarometry of quartz is achievable via the quantification of titanium trace element concentrations when utilizing hyperspectral imaging, allowing a successful deconvolution of the 2.72 eV peak typically associated with titanium.

This information has been sourced, reviewed and adapted from materials provided by Gatan Inc.
For more information on this source, please visit Gatan Inc.