Extensively studied since the 1990s, PNIPAm, Poly(N-isopropyl acrylamide), is a thermally sensitive polymer. PNIPAm shows a phase transition that corresponds to temperature variation thanks to the characteristics of its molecular structure.
As a result of hydrogen bond interaction and the hydrophobic effect, the polymer chains gradually shrink to a collapsed conformation when the temperature exceeds its low critical solution temperature (LCST).
Interestingly, the thermal sensitive property of PNIPAm molecules can be effectively maintained when compounded or copolymerized with other materials, and the phase transition behavior is reversible. A thermal sensitive characteristic such as this holds promise for a range of applications in smart materials and pharmaceuticals design.
A light scattering technique was used in this process in order to characterize the sizes and zeta potentials of a PNIPAm hydrogel with the change of temperature and subsequently to observe and record the impact of the solution environment on its structure.
Experimental
The PNIPAm hydrogel is dispersed in water. 25 °C - 50 °C was set as the measurement temperature range, and via the function of a temperature trend, measurements were performed with a temperature interval of 1 °C, as was a heating-cooling cycle.
60 seconds was set as the temperature equilibration time for every temperature interval, in order to ensure that the measured sample is in thermal equilibrium.
Results and Discussion
Figure 1 depicts that, during the temperature raising process, the particle size of PNIPAm hydrogel decreases when temperature increases in the range of 25 °C - 50 °C, while the count rate gradually increases. The particle size is approximately 700 nm at 25 °C, while the particle size is reduced to around 350 nm when it eventually reaches 50 °C.
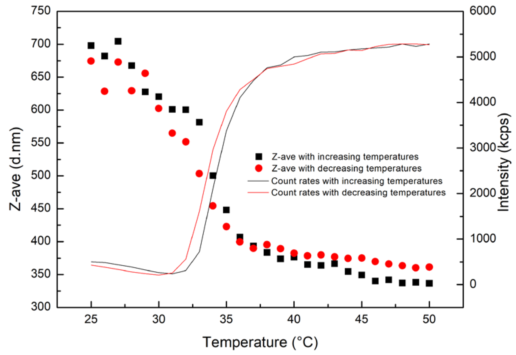
Figure 1. Particle sizes and count rates of PNIPAm hydrogel as a function of temperature. Image Credit: Bettersize Instruments Ltd.
When the surrounding temperature has exceeded a phase transition temperature (which is around 32 °C, as reported in most literature, but it also relies on the colloidal structure), the PNIPAm size decreases upon heating.
Rapid changes would result in its conformation from the unfolded-coil state to the collapsed state from the hydrophobic effect of PNIPAm molecules and the formation of a protein-like hydrogen bond.
An increase in colloidal density and the value of (dn/dc) for suspension is the end result of a gradual increase of count rates from the shrinking of PNIPAm. The count rates are directly proportional to the value of (dn/dc)2.
The assembled behavior in the heating-cooling cycle is reversible, as depicted in Figure 1. The heating and cooling cycle displays the obvious hysteresis of the phase transition. This is due to the fact that PNIPAm needs to absorb energy so that it can create the hydrogen bonds upon heating, while the cooling process occurs as a result of the released energy from the breaking of hydrogen bonds.
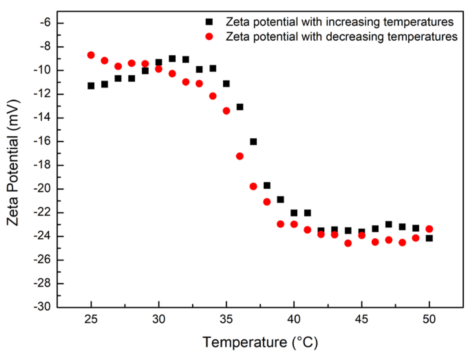
Figure 2. Zeta potentials of PNIPAm hydrogel as a function of temperature. Image Credit: Bettersize Instruments Ltd.
The zeta potentials trend of the sample under different temperatures is displayed in Figure 2. In the detected temperature range, the zeta potentials of PNIPAm hydrogel are negative within the detected temperature range, as illustrated, which means the sample carries negative charges on its surface.
With the elevation of temperature, the amplitude of zeta potentials increases. The zeta potential of PNIPAm is approximately -10 mV at 25 °C, and the potential is increased to approximately - 24 mV when the temperature reaches 50 °C. For both the heating and cooling processes, the zeta potential trend of PNIPAm hydrogel behaves the same.
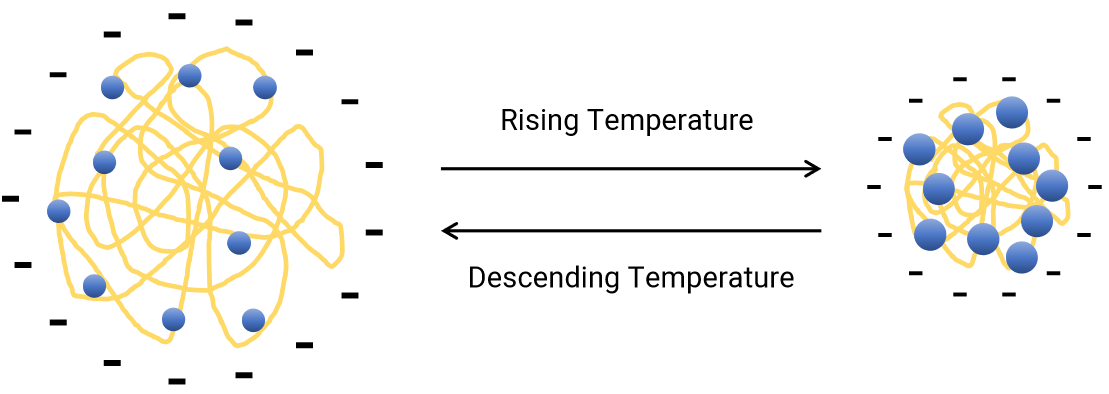
Figure 3. The schematic diagram for the temperature dependence of PNIPAm conformation. Image Credit: Bettersize Instruments Ltd.
Figure 3 displays the temperature dependence of the zeta potential of the PNIPAm sample. The sample, at relatively lower temperatures, displays unfolded conformation, where the surface charge density is relatively lower; however, the particle size decreases with the increasing temperature which leads to an increase in surface charge density along with the zeta potential amplitude.
Conclusion
The process described in this article is that of a thermosensitive PNIPAm sample being characterized by automated measurements of particle sizes and zeta potentials under the programmed temperature change process of the BeNano. Similar behavior was exhibited by the PNIPAm exhibits as with the reported results from most literature.
Measurement efficiency can be significantly improved by the temperature trend measurement of the BeNano, which offers a robust and powerful testing tool for multiple applications.

This information has been sourced, reviewed and adapted from materials provided by Bettersize Instruments Ltd.
For more information on this source, please visit Bettersize Instruments Ltd.
