Conducting polymers refers to polymers that can transmit electrical charge rendering them viable conductor options. These substances integrate metal's electric charge conduction capabilities with the benefits of polymeric capabilities.
Image Credit: Irina Anosova/Shutterstock.com
Various Conducting Polymers and Their Synthesis Methods
Polypyrrole, poly(phenylacetylene), poly(p-phenylene sulfide), poly(p-phenylene), polythiophene, polyfuran, polyaniline, polycarboxylate, and its derivatives are some of the several distinct conductive polymers effectively used in industry. The researcher Hideki Shirakawa was awarded the noble prize in the year 2000 for the development and increase in conductivity by contamination/doping of polyacetylene films.
Chemical oxidizing reactions, electrochemical processing, vapor phase polymerization, geothermal, hydrothermal method, template-assisted, electrospinning, self-assembly, photocatalytic procedures, the inclusion technique, the solid-state process, and plasma gelation were all used to create conducting polymers.
Polyacetylene and its derivatives exhibit multifunctionality. Polyacetylene may be synthesized using a variety of techniques, including catalytic polymerization, non-catalytic polymerization, enzymatic polymerization of other monomers, and precursor-assisted production.
Catalysts such as Ziegler–Natta catalysts or Luttinger catalysts are employed for production in the event of catalyzed polymerization. Polyaniline is among the most intriguing and extensively researched conducting polymers. One of the simplest ways to produce polyaniline is by the oxidation process. Polyaniline is also synthesized through surface modification, in which an aniline monomer is dissolved in an extraction liquid such as toluene.
Polypyrrole is a major conducting polymer that is obtained by utilizing electrochemical synthesis. Poly(p-phenylene) is a large molecule composed of aromatic benzoid cores. The first chemically synthesized poly(p-phenylene) was achieved using a metal bonding process known as the Wurtz–Fittig reaction.
Direct benzene molecule oxidation is commonly used in the manufacture of poly(p-phenylene)s. Polythiophene has been successfully chemically produced using techniques such as direct sol-gel, oxidation production, organometallic bonding process, electro-polymerization, template-assisted formulation, and thermal and solvothermal processes.
Electrical Properties of Conducting Polymers
The electronic band formations of a substance are commonly used to describe its electrical characteristics. The energy differential between the bandgap and the valance band distinguishes between insulators and conductors. The bandgap of intrinsically conducting materials is narrower, and the conduction and valance bands overlap.
All conducting polymers feature conjugated connections in their polymer backbone, which are essential for electron mobility. Undoped virgin polymers can serve as insulators or semiconductors, and conductivity rises as contaminant substance concentration increases. Doping is classified into two types: p-type doping and n-type doping, and the dopants create both positively and negatively charged polarons/bipolarons.
Charge carriers are essential for transmission in the conjugated polymer conduction mechanism. Charge carriers in conduction polymers hop not only in the faulty region but also in interlayer intervals. According to Mott, dielectric properties are affected by temperature and concentration. The Mott model has been used successfully to describe the conduction process in polymorphs and organic polymers, and this theory attempted to investigate the effects of heat on conductance.
Optical Properties
Owing to the existence of p bonds in the polymer backbone, the electron configuration of conjugated polymers is anisotropic and quasi-one-dimensional. Sub-gap photonic changes take place in the polymeric matrix, whereas doping causes charge mobility through an oscillation amplitude shift.
The phenomenon of electroluminescence has been discovered in conducting polymers and was originally documented in poly(p-phenylene vinylene). When conducting polymers are doped with fullerene, they exhibit a unique optical feature, and disubstituted polyacetylene exhibits better photoluminescence quantum yield than undoped or monosubstituted polyacetylene. Optical changes in conducting polymers occur as a result of chemical reactions as well as extrinsic variables such as a strain effect on the polymer matrix and a shift in planarity.
Applications of Conducting Polymers
Polymeric materials are employed in several applications. Ionic conducting polymers are employed in energy applications like lithium batteries. Because of their electrical qualities, relatively high capacitance, excellent wave uptake, strong redox reactivity, and excellent electrochemical behavior, conducting polymers have a wide range of uses.
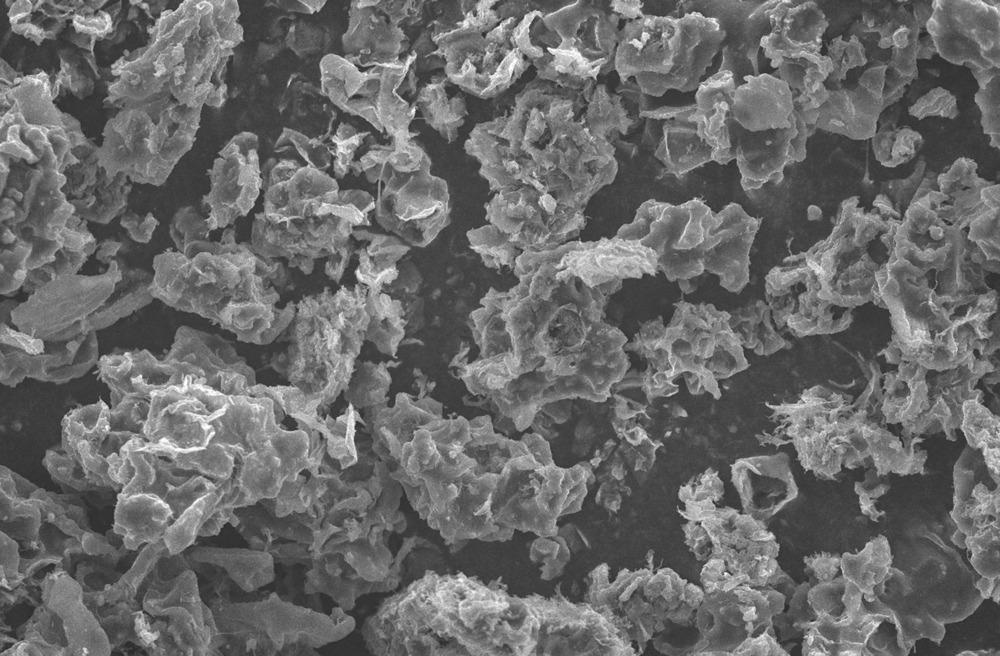
Polythiophene particles with a scanning electron microscope. Image Credit: TinyPhoto/Shutterstock.com
Conducting polymers are available in various morphologies such as films, nanoparticles, hydrogels, rod-like structures, and so on. These various morphologies have varying electronic, biomechanical, and optical characteristics. Polyaniline's mechanical performance is efficiently used in the construction of flexible supercapacitor electrodes due to its ease of manufacture, redox properties, and permeability. Conducting polymers and conductive polymer tinted paints play a critical role in corrosion prevention.
Conducting polymers have gained extensive attention as integral elements of corrosion-resistant coatings due to their capacity to preserve a metal's persistent receptivity via an anodization process paired with O2 reduction on the film's surface. Conducting polymers, particularly polyaniline, also have the characteristic of regulated inhibitor release upon degradation. A chemically produced polyaniline/CdO composite, on the other hand, shows good dye removal ability under UV and solar irradiation.
Conducting polymers such as polyaniline, polypyrrole, and PDOT, have an exceptional capacity to absorb IR while being far less hazardous. As a result, these polymers are commonly utilized in both in vivo and in vitro cell research. Furthermore, because of their excellent photodynamic effectiveness of 48.5 percent, synthesized polyaniline hybrids have a promising future in photothermal treatments due to their high quantity of strong NIR absorbance, ideal size, decent water distribution, and high photothermal efficacy of 48.5 percent.
Research Findings
The latest research by Mr. Hagler and his team published in the journal Flexible and Printed Electronics focuses on the development of stretchable bioelectronics made up of conducting polymers. The research team created highly flexible poly 3,4-ethylene dioxythiophene: polystyrene sulfonate (PEDOT: PSS) sheets imprinted on thermoplastic polyurethane (TPU) surfaces.
The gadget conductivity's durability at high stresses of up to 600 percent has been thoroughly demonstrated. On a substrate material, the printed PEDOT: PSS layer permitted the creation of printed organic electrochemical semiconductors with an ON/OFF ratio of 450. By directly connecting a printable PEDOT: PSS-based sensor on TPU with the skin surface, the team was also able to gather physiological data by monitoring skin conductance arising from differences in sweat volume. Stretchy printed PEDOT: PSS films on TPU offer an easy way to create very robust stretchable detectors for bioelectronic applications.
In short, conducting polymers are useful for various purposes owing to their robust properties.
References and Further Reading
Kim, Chihyeong, et al. 2022. Flexible and stretchable printed conducting polymer devices for electrodermal activity measurements. Flexible and Printed Electronics. 7(1). 014008 Available at: https://iopscience.iop.org/article/10.1088/2058-8585/ac4d0f
Namsheer, K., and Chandra Sekhar Rout. 2021. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Advances 11(10). 5659-5697. Available at: https://pubs.rsc.org/en/content/articlelanding/2021/ra/d0ra07800j
Criado-Gonzalez, Miryam, et al. 2021. Additive manufacturing of conducting polymers: recent advances, challenges, and opportunities. ACS Applied Polymer Materials 3(6). 2865-2883. Available at: https://pubs.acs.org/doi/10.1021/acsapm.1c00252
Wang, Tao, et al. 2021. Nanoscale engineering of conducting polymers for emerging applications in soft electronics. Nano Research 14(9). 3112-3125. Available at: https://link.springer.com/article/10.1007/s12274-021-3515-8
Guo, Xugang, and Antonio Facchetti. 2020. The journey of conducting polymers from discovery to application. Nature Materials 19(9). 922-928. Available at: https://www.nature.com/articles/s41563-020-0778-5
Tajik, Somayeh, et al. 2020. Recent developments in conducting polymers: Applications for electrochemistry. RSC Advances 10(62). 37834-37856. Available at: https://pubs.rsc.org/en/content/articlelanding/2020/ra/d0ra06160c
Bazli, Leila, et al. 2020. Application of composite conducting polymers for improving the corrosion behavior of various substrates: A Review. Journal of Composites and Compounds. 2(5). 228-240. Available at: https://www.jourcc.com/index.php/jourcc/article/view/jcc247
Stejskal, Jaroslav. 2020. Conducting polymers are not just conducting: a perspective for emerging technology. Polymer International 69(8). 662-664. Available at: https://onlinelibrary.wiley.com/doi/10.1002/pi.5947
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.