Sponsored by TOFWERKReviewed by Emily MageeJun 21 2022
The primary technologies used for etch process characterization and monitoring are optical spectroscopy and mass spectrometry. Though desirable in production settings because of their non-invasive nature, optical techniques are only applicable in specific cases and cannot address all chemistries and materials.

Image Credit: TOFWERK
Mass spectrometry, by contrast, is a powerful technique for the analysis of any process gas. Careful consideration of differences in pressure, reaction time scales and process flow is essential for integrating mass spectrometers. Previously, these constraints have been addressed by the use of quadrupole mass spectrometers (residual gas analyzers, RGAs). As the semiconductor industry incorporates ever-more materials in highly complex stacks for the design of the next-generation chips, however, a situation is created by the limited mass range and low mass resolution and sensitivity of RGAs in which they are simply no longer able to master the challenges of integration.
The Process Gas Analyzer from TOFWERK
The pgaTOF from TOFWERK is an electron ionization time-of-flight mass spectrometer and was developed specifically to overcome the limitations of RGAs. Simultaneous detection of all ionized species with isotopic resolution, greater than 105 dynamic range and a mass spectral acquisition rates up to 1000 Hz are provided by the pgaTOF. In order to guide process control action and to inform analytics and process intelligence, all process gas and etch products are monitored. Optimizing flow rates and ratios, monitoring substrate temperature, or detection of process excursion from optimal conditions or malfunctions are all included in immediate modifications of process conditions.
Spontaneous and Plasma Enhanced Etching of Silicon
Two well-studied, semiconductor-relevant chemistries were investigated in this proof-of-concept etch experiment with the pgaTOF – the first, spontaneous Si etching in XeF2 and the second plasma etching of Si in a CF4/O2 chemistry.
Through a ~1 meter bellow, the pgaTOF was connected to the process reactor. The pgaTOF was connected directly to the reactor chamber in the spontaneous etch case. The pgaTOF was connected at the rough pump level for the plasma etch system. The process pressures were varied from approximately 3-400 mTorr (≈ mbar), with sample temperatures ranging from 20 C to just over 200 C.
The expected etch reactions for both XeF2 and CF4/O2 conditions are shown below. It is important to note that non-volatile carbon compounds accumulate on the sample surface in a pure CF4 plasma, hindering surface chemistry and generating lower etch rates and rough surfaces. Adding O2 to the etch gas results in the reaction of these non-volatile compounds and the formation of CO2, greatly increasing the etch rate.
Spontaneous Etching of Si in XeF2
Si Etching with XeF2
(1) XeF2 -> XeF + F -> Xe + F2 ; Si + 2 F2 -> SiF4
Plasma Etching in a CF4 and CF4/O2 chemistry
Below, si etch reactions are shown for a pure CF4 plasma (2) and (3) for a CF4/O2 mixture
(2) Si + CF4 -> SiF4 + C
(3) Si + CF4 + O2 -> SiF4 + CO2
Experimental Results
Spontaneous Etch of Si with XeF2
Si samples (3 cm2) were inserted into an atomic layer deposition (ALD) reactor, which was fitted with a XeF2 source with variable pulsing capability and using Ar as a carrier gas.
Figure 1 displays all relevant species and their time variation as a XeF2 pulse is introduced in the reactor.
In Figure 2 the same data with varied pulse width can be seen.
The detailed analysis of data depicted in Figure 2 shows the fact that real-time optimization of the etch gas flux and resulting by-products is possible. This points out the ability to determine the optimum time needed to purge out the by-products prior to initiating the next process cycle, which is similar to the ALD case, and more importantly in an atomic layer etching process.
Furthermore, detection of a transition from an etch-rate-limited mode to loading-limited conditions is permitted during process development and optimization. It is important to note that large signal counts are measured for all species, despite the very small sample size. Etch rates significantly smaller than one monolayer per second are expected for these process conditions, on the basis of both previous results and published literature for this etch chemistry. Finally, with the time-of-flight technology of the pgaTOF all compounds – whether known in advance or not – are recorded and stored for off-line analysis, understanding and forensics.
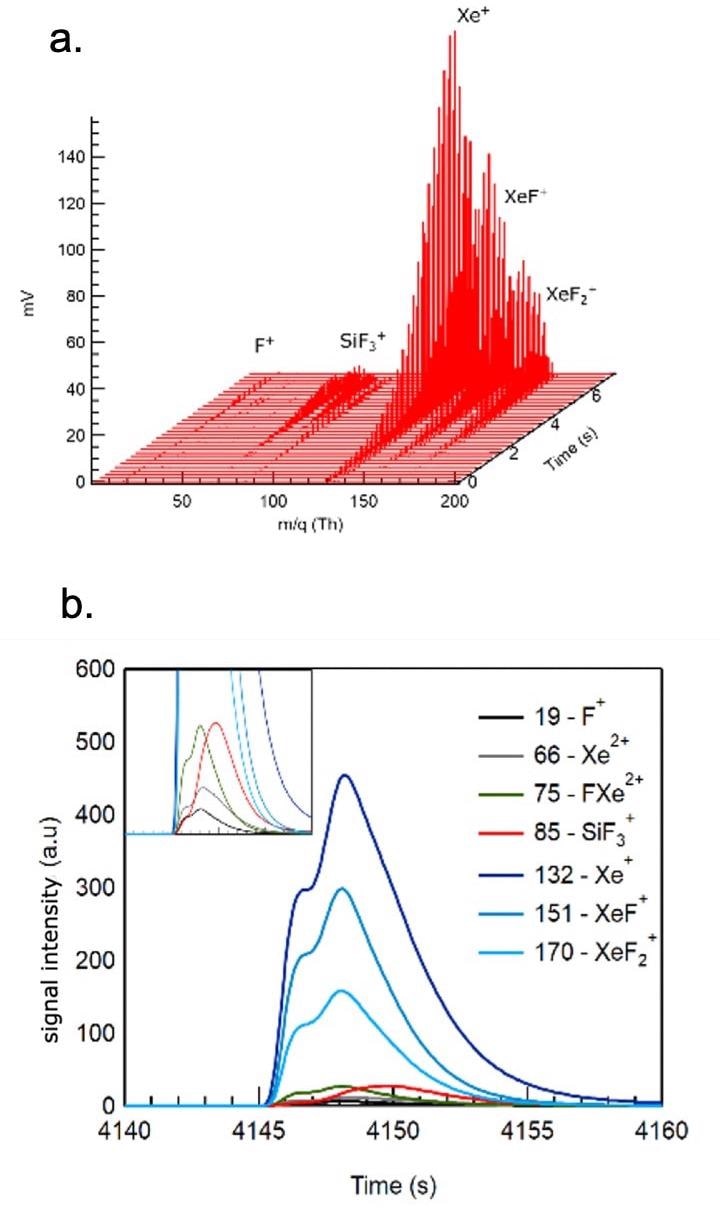
Figure 1. Spontaneous etching of silicon with XeF2. Figures 1a (top) and 1b (bottom) show the time evolution of the most abundant species as the etch gas is introduced in a pulsed mode (100 ms and 0.5 mbar in an Ar carrier gas). Image Credit: TOFWERK
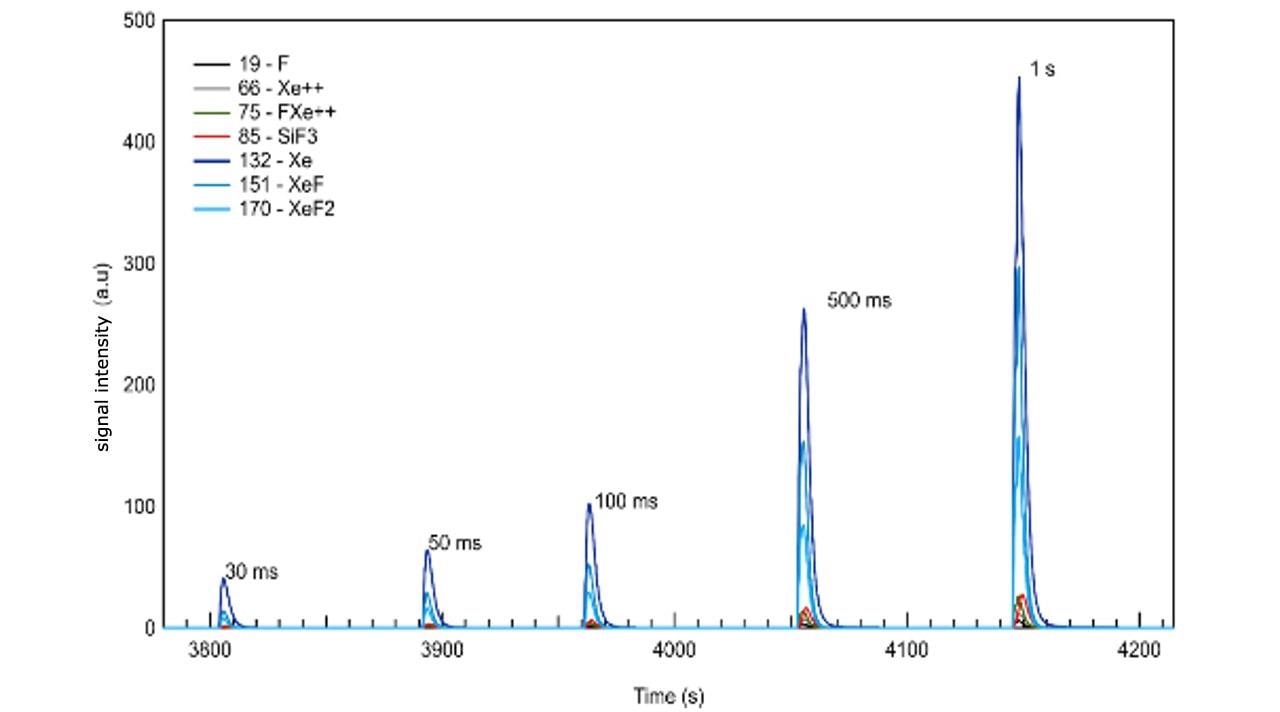
Figure 2. Spontaneous etching of silicon with XeF2 showing the signal time variation of the most abundant species as the etch gas is introduced using pulses of different duration (0.5 mbar in an Ar carrier gas). Image Credit: TOFWERK
Reactive Ion Etching of Si in a CF4/O2 Chemistry
Both a downstream microwave plasma etcher and a standard reactive ion etch chamber (RIE) were used to conduct these experiments. Only cm-sized Si substrates were utilized in both cases. Varying pressures and substrate temperatures. were used to perform these runs using both pure CF4 and CF4/O2 etch mixtures.
Figure 3 shows typical low-mass data for a pure CF4 RIE etch run. The collected spectrum is dense with peaks, despite the simple etch chemistry and the most basic substrate.
Figure 4 depicts the time dependence of relevant species in the mass spectrum as the plasma is turned on and off and the O2 content in the CF4 plasma increased.
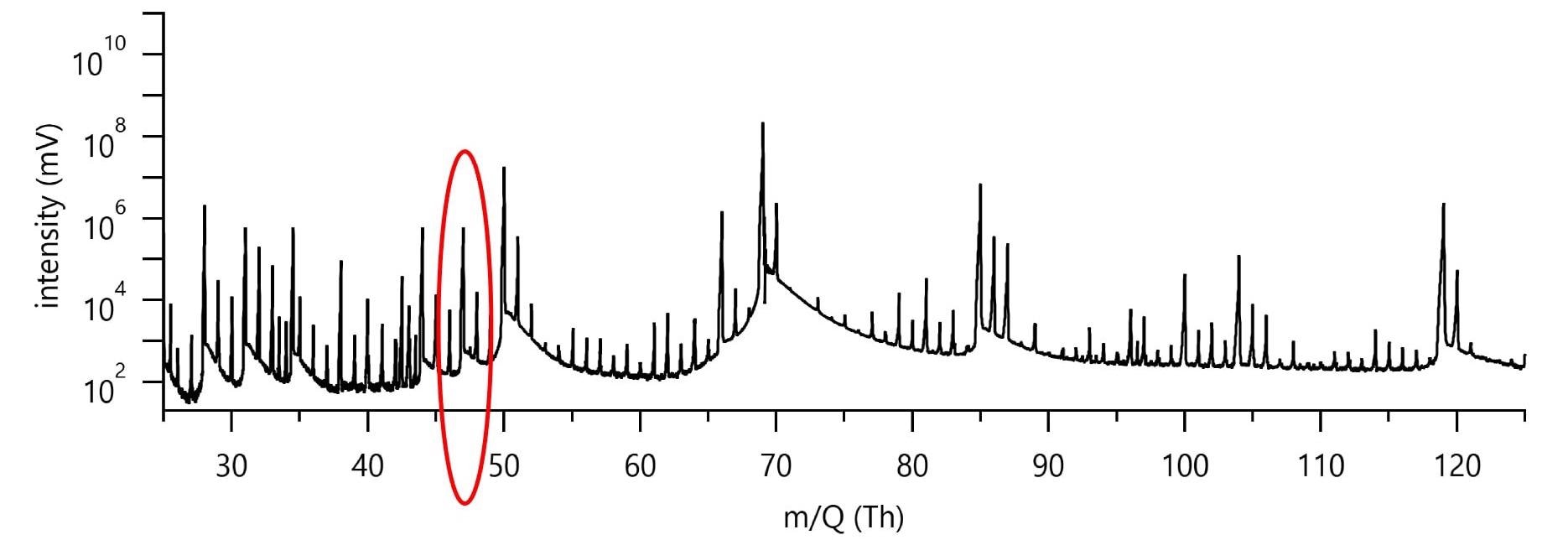
Figure 3. Figure 3 shows typical spectrum during a Si etch run in a pure CF4 plasma (400 W, 100 sccm CF4). Image Credit: TOFWERK
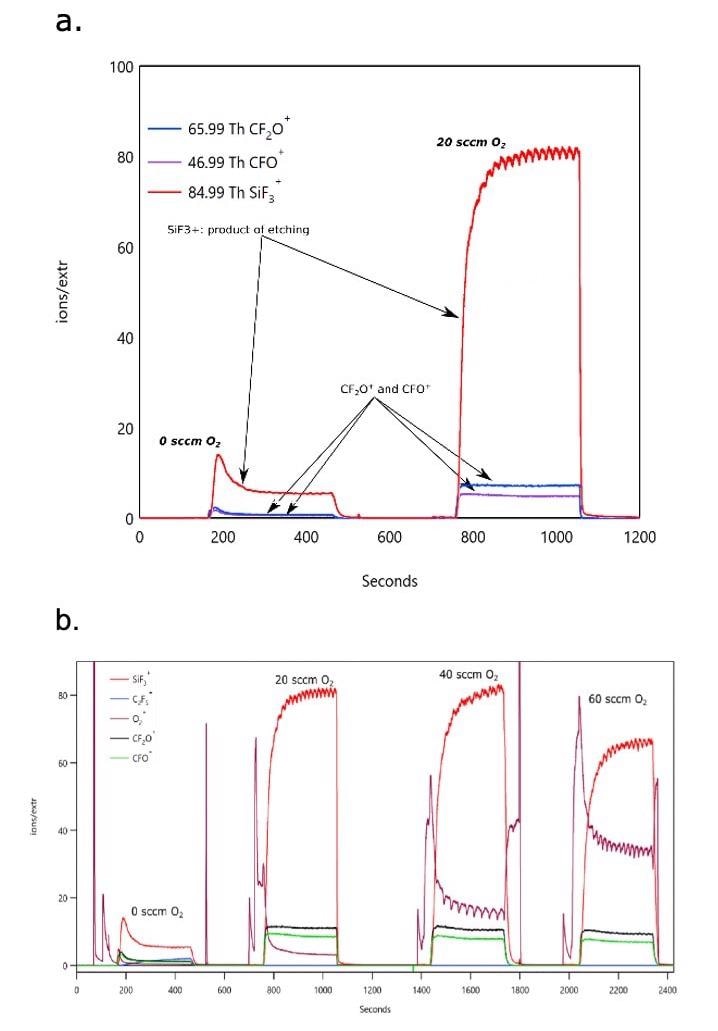
Figure 4. Figure 4 shows time evolution of plasma and etch product species that takes place during a CF4 RIE process showing the effect of O2 addition on the etch rate. a. (top) Power: 400 W, 100 sccm CF4, variable flow of O2 (0, 20 sccm), b. (bottom) Power: 400 W, 100 sccm CF4, variable flow of O2 (0, 20, 40 and 60 sccm). Image Credit: TOFWERK
The abundance of etch products increases significantly with O2 content, as anticipated, but ultimately reaches its maximum at ~ 40 sccm (figure 3b) and beyond that value begins to decrease due to reactive species dilution. A further note of interest is that, as a result of small variations in O2 flow rates, measurable oscillations in the etch product signal can be observed which become significant as it moves beyond the optimum O2 concentration (max etch rate) and travels into the reactive species deficient regime. Such behavior is probably not critical in thick layer etching but would need to be corrected for when processing nanostructured devices.
pgaTOF Records All Compounds and Isotopic Distributions with High Mass Accuracy
Even the simplest etch chemistry (i.e., Si in a CF4 plasma) generates complex mass spectra where assignment of the observed peaks is not always simple or even possible, as has been demonstrated in this proof-of-principle example.
It is therefore important to illustrate the challenge to identifying the peak at 47 Th, as highlighted in Figure 3 within the red circle, to a specific etch gas or product species. The only possible species should have combination of the input etch gas and substrate atomic constituents, C, F, and Si for the pure CF4 plasma chemistry and substrate at play.
The etch product SiF+, at 46.974 Th (383 ppm mass deviation from measured peak) as a possible assignment is provided by use of the composition finder function of the pgaTOF’s software. However, full mass spectral acquisition facilitates the detection of a small but measurable O2 signal which extends the possible species to oxygen-containing compounds.
The composition finder gives us CFO+ at 46.992 Th (0.2 ppm mass deviation from measure peak) as another match.
For better understanding of the etch chemistry, assigning the correct specie to the peak at 47 Th in this case is paramount - and more importantly, is key to determining whether process integrity is acceptable or not; SiF+ being an expected etch product but CFO+ an unexpected impurity related specie.
Figure 5 condenses these results. The isotopic distribution is examined for the two possible species and their match to the experimental pattern in order to further improve the mass assignment. The isotopic distribution for both species is shown by Figure 5.b and it is clear that only CFO+ presents a good visual match.
Having established these two key observations, the peak at ~ 47 Th can therefore be unambiguously assigned to CFO+, which can indicate a possible leak (O2 flow meter or external) or other contamination sources.
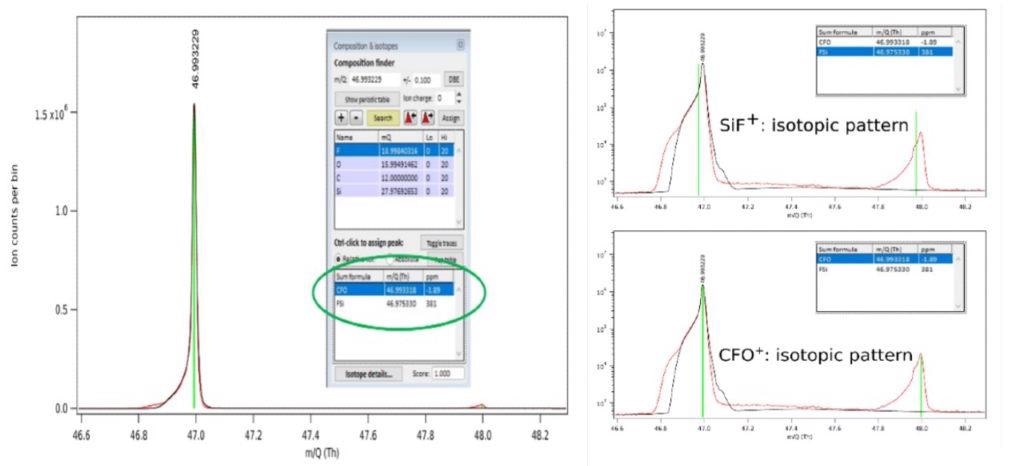
Figure 5. Identification of peak at 47 Th in the spectrum acquired during Si etch in a pure CF4 plasma using the instrument’s high mass accuracy (left panel) and measured isotopic distribution peaks with expected position for the two possible species (right panel). Image Credit: TOFWERK
Conclusion
To conclude, connecting a mobile pgaTOF instrument to an etch reactor in a minimally invasive manner has been demonstrated to provide important process insights for even the simplest etch chemistry (CF4/O2) and most basic substrate (Si).
The real-time monitoring of all volatile compounds with high mass accuracy and sensitivity and sub-second detection of process changes was demonstrated. In unambiguous species assignment, the pgaTOF’s ability to measure masses with very high accuracy and resolution was shown to be critical. On-line reactor health assessment and off-line data analysis for process optimization and failure analysis was facilitated by intrinsic measurements of all species.

This information has been sourced, reviewed and adapted from materials provided by TOFWERK.
For more information on this source, please visit TOFWERK.