Sponsored by PerkinElmerReviewed by Olivia FrostSep 15 2022
Also known as palmitoyl alcohol or 1-hexadecanol, Cetyl alcohol is a long-chain aliphatic alcohol. It is a common additive frequently found in various items, including foods, pharmaceuticals, cosmetics, personal care products (e.g., creams and lotions), and industrial fuels.
Cetyl alcohol has several different uses: as an emulsifier, a thickening agent, a lubricant, a moisturizer, and a flavoring agent in product formulations. Cetyl alcohol is safe for human consumption, both orally and via contact with the skin.1 Many studies have found that minor impurities in cetyl alcohol might cause dermatitis.2
The United States Pharmacopoeia (USP) has outlined a chemical purity assay within the USP-National Formulary (NF) Cetyl Alcohol monograph3 to ensure cetyl alcohol quality.
The monograph outlines various procedures to assess instrument performance, measure cetyl alcohol content, and identify common aliphatic alcohol impurities (stearyl alcohol, lauryl alcohol, myristyl alcohol, and oleic alcohol) and other unidentified contaminants using gas chromatography/flame ionization detection (FID).

PerkinElmer GC 2400 System. Image Credit: PerkinElmer
The performance of the PerkinElmer GC 2400™ System with FID is outlined within this article for the continued offering of enhanced compound resolution and highly repeatable injections at the same time as analyzing residual impurities in cetyl alcohol.
Instrumentation
An FID and PerkinElmer Elite 225 analytical column were used to configure the GC 2400 System. This was used to analyze cetyl alcohol content and identify impurities. Users can automatically generate suitability data through the PerkinElmer SimplicityChrom™ Chromatography Data System (CDS) Software.
Experimental
Table 1. Chromatography conditions. Source: PerkinElmer
| System |
Part Numbers |
| Gas Chromatograph |
PerkinElmer GC 2400 System |
-- |
| Injector |
Capillary Split/Splitless (CAP) with PerkinElmer AS 2400™ Liquid Sampler |
-- |
| Advanced Green Inlet Septum |
N9306218 |
| Green Focus liner |
N6502041 |
| 5 uL Autosampler Syringe |
N6402556 |
| Detector |
Flame Ionization Detector (FID) |
-- |
| Grade 5 Hydrogen, 35 ml/minute |
-- |
| Grade 5 Air, 400 ml/minute |
-- |
| Grade 5 Nitrogen, 25 ml/minute |
-- |
| Gas Filters |
Triple Filter (Hydrogen & Nitrogen) |
N9306110 |
| Moisture/Hydrocarbon Trap (Air) |
N9306117 |
| Analytical Column |
Elite 225; 30 m x 0.25 mm x 0.25 μm |
N9316177 |
| Software |
SimplicityChrom CDS Software |
|
| Conditions |
|
| Carrier |
Grade 5 Hydrogen, 2 ml/minute |
| Septum Purge |
3 ml/minute |
| Split |
5:1 ratio* |
| Injection Volume |
0.5 μL |
| Injector Temp. |
270 °C |
| Detector Temp |
280 °C |
| Oven |
60 °C for 0 minutes, 20 °C/minute to 180 °C,
10 °C/minute to 220 °C, hold for 5 minutes |
*Note: a 5:1 split ratio was used to carry out all tests. This slightly differs from the monograph, which recommends a split of 100:1 for the assay and a split of 5:1 for the impurity test and resolution check.
Millipore Sigma (Burlington, MA, provided high purity (> 99 wt%) cetyl alcohol, stearyl alcohol, lauryl alcohol, oleyl alcohol, and myristyl alcohol). Millipore Sigma provided the 1-pentadecanol internal standard and 200 proof ethanol diluent. An online cosmetics vendor provided the natural commercial cetyl alcohol sample.
The procedures outlined in the PerkinElmer Capillary Column Quick Care Guide were followed in the conditioning of the analytical column. Approximately 100 mg of 1-pentadecanol was diluted into 100 ml of 200-proof ethanol in a volumetric flask to prepare 1 mg/ml of internal standard solution.
The solution was ultrasonicated at 50 °C for five minutes, after which all solids had dissolved.
Approximately 10 mg of lauryl, oleyl, stearyl, myristyl and cetyl alcohol were weighed in the same 10 ml volumetric flask to prepare the standard solution. To dissolve the solutes, 10 ml of internal standard solution were added, producing a mixture containing approximately 1 mg/ml of each component.
The standard solution was further diluted with ethanol to produce the resolution check solution, making a final test mix concentration of approximately 0.05 mg/ml. By dissolving around 10 mg of the sample into 10 mL of internal standard solution, the commercial sample was prepared.
A GC 2400 System using SimplicityChrom CDS Software was used to perform this analysis. Each solution was analyzed five times. Streamlined data acquisition and processing is facilitated by the straightforward software interface. The required information for fulfillment of the monograph is automatically generated by the software’s system suitability function.
GC-FID: Limit of Cetyl Alcohol and Aliphatic Alcohol Impurities According to USP
Results & Discussion
USP Retention Time
Given that the hydrocarbons’ signal is otherwise indistinguishable, retention time identification is critical when using flame ionization detection.
Reliable identification of compounds by retention time (RT), as shown in Table 2, is permitted by the GC 2400 System’s advanced Pneumatic Pressure Controller (PPC) is capable of highly repeatable separations. Figure 1A shows a relative standard deviation of 0.01% - 0.02% for the average RT of each compound.
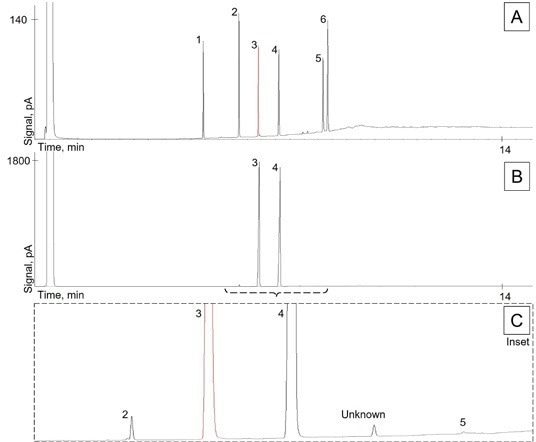
Figure 1. Chromatograms of (A) the resolution check solution, (B) the commercial cetyl alcohol sample, and (C) the inset of commercial sample highlighting low-level impurities. Compounds—1: Lauryl Alcohol; 2: Myristyl Alcohol; 3: 1-Pentadecanol internal standard; 4: Cetyl Alcohol; 5: Stearyl Alcohol; 6: Oleyl Alcohol. Image Credit: PerkinElmer
Note: Sample B is at approx. 20x concentration versus resolution check solution, Sample A.
Table 2: Table 2 displays retention time (RT) and a relative retention time (RRT) repeatability study of the long-chain aliphatic alcohols within the resolution check solution. The RRT internal standard is 1-pentadecanol. Comparing internal RRT values against those in the USP monograph, relative percent difference (RPD) is provided (equation shown below).
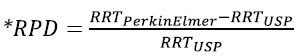
Table 2. Retention time (RT) and relative retention time (RRT) repeatability study of the long-chain aliphatic alcohols in the resolution check solution. The RRT internal standard is 1-pentadecanol. Relative percent difference (RPD) is provided comparing internal RRT values against those in the USP monograph, the equation is shown below. Source: PerkinElmer
| |
Lauryl Alcohol |
Myristyl Alcohol |
1-Pentadecanol |
Cetyl Alcohol |
Stearyl Alcohol |
Oleyl Alcohol |
| Run 1 RT (min) |
5.56 |
6.57 |
7.11 |
7.69 |
8.94 |
9.08 |
| Run 2 RT (min) |
5.56 |
6.57 |
7.11 |
7.70 |
8.94 |
9.08 |
| Run 3 RT (min) |
5.56 |
6.57 |
7.11 |
7.69 |
8.94 |
9.07 |
| Run 4 RT (min) |
5.55 |
6.56 |
7.11 |
7.69 |
8.94 |
9.07 |
| Run 5 RT (min) |
5.55 |
6.56 |
7.11 |
7.69 |
8.94 |
9.07 |
| Avg. RT (min) ± |
5.55 ± |
6.56 ± |
7.11 ± |
7.69 ± |
8.94 ± |
9.07 ± |
| RSD |
0.02% |
0.02% |
0.02% |
0.02% |
0.01% |
0.01% |
| Run 1 RRT |
0.78 |
0.92 |
1.00 |
1.08 |
1.26 |
1.28 |
| Run 2 RRT |
0.78 |
0.92 |
1.00 |
1.08 |
1.26 |
1.28 |
| Run 3 RRT |
0.78 |
0.92 |
1.00 |
1.08 |
1.26 |
1.28 |
| Run 4 RRT |
0.78 |
0.92 |
1.00 |
1.08 |
1.26 |
1.28 |
| Run 5 RRT |
0.78 |
0.92 |
1.00 |
1.08 |
1.26 |
1.28 |
| Avg. RRT ± |
0.78 ± |
0.92 ± |
n/a |
1.08 ± |
1.26 ± |
1.28 ± |
| RSD |
0.01% |
0.01% |
|
0.01% |
0.01% |
0.01% |
USP Literature
RRT2
RPD* |
0.79
1.13% |
0.93
0.75% |
1.00
n/a |
1.09
0.75% |
1.25
-0.57% |
1.28
0.32% |
For each component against the 1-pentadecanol internal standard, the USP monograph provides relative retention times (RRT); literature values are shown in Table 2. Between internal and external (USP) values, excellent accuracy was obtained: approximately 1% or less. With an average RRT standard deviation of 0.01% for each compound, excellent precision was also obtained.
USP Resolution Check
With an Elite 225 capillary column, the performance of the GC 2400 System greatly surpasses the USP method criteria for compound resolution. A myristyl alcohol and 1-pentadecanol resolution of a minimum of 15, a cetyl and stearyl alcohol resolution of at least 30, and a stearyl and oleyl alcohol resolution of at least 2 in the resolution check solution are required by this method. With improvements to each compound resolution of 45% (21.71), 33% (39.99), and 100% (3.99), respectively, the results in Table 3 show that these values were exceeded.
Table 3: Table 3 shows the results of the resolution study based on the USP resolution acceptance criterion. It is proven by these results that the PerkinElmer system outperforms each acceptance criterion.
Table 3. Results of the resolution study based on USP resolution acceptance criterion. Results prove that the PerkinElmer system outperforms each acceptance criterion. Source: PerkinElmer
| |
Myristyl:1- Pentadecanol |
Cetyl:Stearyl |
Stearyl:Oleyl |
| Run 1 Resolution |
21.69 |
39.08 |
3.93 |
| Run 2 Resolution |
21.90 |
40.10 |
4.02 |
| Run 3 Resolution |
21.57 |
41.37 |
4.09 |
| Run 4 Resolution |
21.83 |
39.59 |
3.91 |
| Run 5 Resolution |
21.56 |
39.81 |
3.99 |
Avg. Res. ±
RSD |
21.71 ±
0.70% |
39.99 ±
2.15% |
3.99 ±
1.82% |
| USP Res. Criterion |
≥ 15 |
≥ 30 |
≥ 2 |
| % Improvement |
45% |
33% |
100% |
USP Asymmetry
Both 1-pentadecanol internal standard and cetyl alcohol must express a tailing factor between 0.80 (peak fronting) and 1.80 (peak tailing) in the standard solution, according to the USP asymmetry criterion. The ideal asymmetry has a value of 1.00 exactly. The near-perfect asymmetry obtained by the PerkinElmer GC 2400 System with Elite 225 column is shown in Table 4, with an average tailing factor of 1.04 for 1-pentadecanol and 1.02 for cetyl alcohol.
Table 4. Tailing factors (TF) for internal standard (1-pentadecanol) and target compound (cetyl alcohol) in standard solution. Source: PerkinElmer
| |
1-Pentadecanol |
Cetyl Alcohol |
| Run 1 TF |
1.03 |
1.01 |
| Run 2 TF |
1.01 |
1.03 |
| Run 3 TF |
1.05 |
1.03 |
| Run 4 TF |
1.06 |
1.02 |
| Run 5 TF |
1.04 |
1.04 |
| Avg. TF ± RSD |
1.04 ± 1.70% |
1.02 ± 1.25% |
| USP TF Criterion |
0.8 to 1.8 |
0.8 to 1.8 |
Cetyl Alcohol
USP Area Ratio
Assessment of the analytical repeatability using the area ratio of cetyl alcohol to 1-pentadecanol in the standard solution is required by the USP monograph. The method requires a system suitability threshold of no more than 1% RSD in this area ratio. As is shown by the results in Table 5, this acceptance criterion has been met.
Table 5: Area ratio (AR) repeatability shows that the PerkinElmer™ system meets the USP monograph requirement.
Table 5. Area ratio (AR) repeatability shows that the PerkinElmer™ system meets the USP monograph requirement. Source: PerkinElmer
| |
Cetyl:1-Pentadecanol |
| Run 1 AR |
1.00 |
| Run 2 AR |
1.00 |
| Run 3 AR |
1.02 |
| Run 4 AR |
1.00 |
| Run 5 AR |
1.01 |
| Avg. AR + RSD |
1.01 ± 0.94% |
| USP AR Criterion |
≤ 1.0% RSD |
Cetyl:1-Pentadecanol
The following equation was used to sample cetyl alcohol purity: where R is the cetyl alcohol to 1-pentadecanol area ratio and C is the cetyl alcohol concentration in mg/ml. The monograph permits a purity of 90% to 102% in the sample. A cetyl alcohol purity of 99% was determined for the commercial sample.

USP Organic Impurity Test
According to Organic Purity Test 1 procedures, our sample of natural cetyl alcohol was analyzed. Figure 1B was used to present the sample chromatogram. Aliphatic alcohol impurities to constitute up to 10% of the total peak area are permitted by the USP method, whereas unidentified peaks may constitute up to 1%.
Our commercial product meets these criteria, based upon the results in Table 6, with a total aliphatic alcohol impurity of 0.76% and an unidentified impurity of 0.41%. As delineated in the method, all other minor peaks were beneath the threshold of 0.05% composition and therefore were excluded from this calculation. A zoomed-in perspective of impurity peaks near the chromatogram’s baseline is shown by Figure 1C.
Table 6: Excluding solvent and internal standard contributions, Table 6 shows the peak area % composition of the commercial sample.
Table 6. Peak area % composition of the commercial sample, excluding solvent and internal standard contributions. Source: PerkinElmer
| |
Myristyl Alcohol |
Cetyl Alcohol |
Stearyl Alcohol |
Unknown Peak |
| Sample 1 Area |
0.72% |
98.78% |
0.08% |
0.41% |
| Sample 2 Area |
0.69% |
98.84% |
0.05% |
0.41% |
| Sample 3 Area |
0.71% |
98.82% |
0.06% |
0.42% |
| Sample 4 Area |
0.75% |
98.77% |
0.07% |
0.41% |
| Sample 5 Area |
0.70% |
98.83% |
0.06% |
0.41% |
Avg. ±
RSD |
0.70% ±
3.24% |
98.83% ±
0.03% |
0.06% ±
16.51% |
0.41% ±
0.78% |
Conclusion
The USP-NF Cetyl Alcohol monograph is essential for determining impurities in cetyl alcohol additives. The GC 2400 System with FID Detector and Elite 225 column was able to meet or exceed system suitability criteria as outlined in the method, displaying consistent retention times for all compounds and repeatable, precise sample injections for the USP compliant analysis of residual impurities in cetyl alcohol.
With a compound resolution of 133% to 200% of the target value, the resolution of target components has a particularly high performance. Even in high sample throughput laboratories, the performance shows high repeatability giving ultimate confidence for executing this analysis. The SimplicityChrom CDS Software was used to perform this data acquisition and analysis, which provides an intuitive, customizable user experience with multi-functionality and accessibility options.
Versatility and portability are provided by the detachable touchscreen, which optimizes time and, ultimately, lab productivity.
References
- Chapter 5: Final Report on the Safety Assessment of Cetearyl Alcohol, Cetyl Alcohol, Isostearyl Alcohol, Myristyl Alcohol, and Behenyl Alcohol. May 1, 1988. Journal of the American College of Toxicology, 7(3): 359-413.
- Komamura, H; Doi, T; Inui, S; Yoshikawa, K (1997). "A case of contact dermatitis due to impurities of cetyl alcohol". Contact Dermatitis. 36 (1): 44–6. doi:10.1111/j.1600-0536.1997.tb00921.x. PMID 9034687.S2CID 23444831.
- U.S.P. USP-NF Cetyl Alcohol, November 1, 2020.

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.