Sponsored by Xenocs SASReviewed by Louis CastelOct 17 2022
The global COVID-19 pandemic highlighted the necessity of accelerating various stages of drug development, lowering drug candidate attrition rates, and establishing reliable testing processes to guarantee that only stable and safe formulations reach the market.
With its robust and fast method for examining the structure, morphology, and interactions in the involved systems, Small Angle X-Ray Scattering (SAXS) can help overcome many of these difficulties.
This white paper describes how research, development and quality control are assisted during various stages of drug and vaccine development by SAXS and WAXS (Wide-Angle X-Ray Scattering).
This document also outlines how SAXS can serve as a fingerprint technique for troubleshooting, manufacturing monitoring and quality control. Furthermore, it explores how high-throughput screening accelerates preclinical development using examples from peer-reviewed scientific literature and interviews with academic and industrial partners.
The SAXS analysis of viruses is also discussed, as is the role SAXS plays in drug and vaccine target development. The timeframe for drug development is summarized in the figure below.
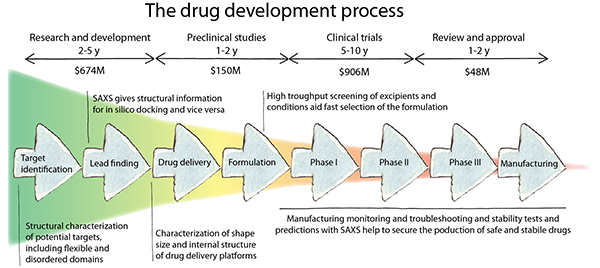
Image Credit: Xenocs
Why SAXS?
The method is similar to light scattering; however, the shorter wavelength of X-rays extends the probe length scale to the molecular level, is less prone to artefacts, and provides access to various parameters.
Laboratory SAXS is common due to advancements made in the last ten years. Previously, however, pioneering research relied on large facilities where synchrotron radiation could be utilized as the X-ray source.
SAXS is an established component of the structural biology toolbox that is used to characterize the size, shape and composition of proteins (including antibodies and antigens), protein complexes, DNA and RNA. SAXS has attracted increased attention due to its application in further pharmaceutical areas, such as formulation.
The principal advantages of SAXS and WAXS are:
- Near–native state measurements in solution
- Both dynamic and static sample manipulation are feasible (e.g., temperature, shear, humidity)
- Measurements can be done in-house
- Measurements can be automated and performed with high-throughput
- Supplements high-resolution structural determination tools (e.g., NMR, Cryo-EM, etc.)
- Suitable for all types of samples (i.e., liquid, paste, solid)
- Can be done in a wide concentration range
- No tedious sample preparation is required
- Low sample volumes of down to 5 μl
- Statistically relevant results can be obtained with one measurement
- Suitable for quality control, R&D, and troubleshooting
- Suitable framework for use in conjunction with in silico modeling
WAXS and SAXS are carried out in a near-native sample environment, avoiding time-consuming sample preparation while providing access to the solution structure. This gives unique insights into structural information about intrinsically flexible and disordered domains in proteins and genetic material.
Laboratory SAXS/WAXS equipment dedicated solely to the requirements of structural biology for drug development, are commercially available. These include automated and high-throughput solutions with low sample consumption.
The rapid and unique characterization from X-ray scattering contributes to a shorter time-to-market and a better understanding of the structural properties of new drugs and vaccines.
Click here to gain access to the complete white paper

This information has been sourced, reviewed and adapted from materials provided by Xenocs.
For more information on this source, please visit Xenocs.