Sponsored by Xenocs SASReviewed by Louis CastelOct 17 2022
Silks are popular natural protein fibers spun by spiders, silkworms, and other insects. They have been utilized in the textile industry for centuries due to their exceptional toughness and strength.
The silk of the domestic silk moth Bombyx mori (B. mori) has been widely studied due to its potential application as a biomedical material.1, 2 Two proteinous polymers3 make up silk fibers, as seen in Figure 1:
- Fibroin consists of a hydrophilic light chain (FibL) and a hydrophobic heavy chain (FibH). The dominant FibH consists of large repetitive units of glycine (GLY) and alanine (ALA) that fold into antiparallel – sheet crystalline structures which in turn are organized into a hydrophilic amorphous matrix that is produced by FibL.
- Sericin is a gum-like hydrophilic antigenic protein that forms the outer layer of the coating which holds the fibroin fibrils together.
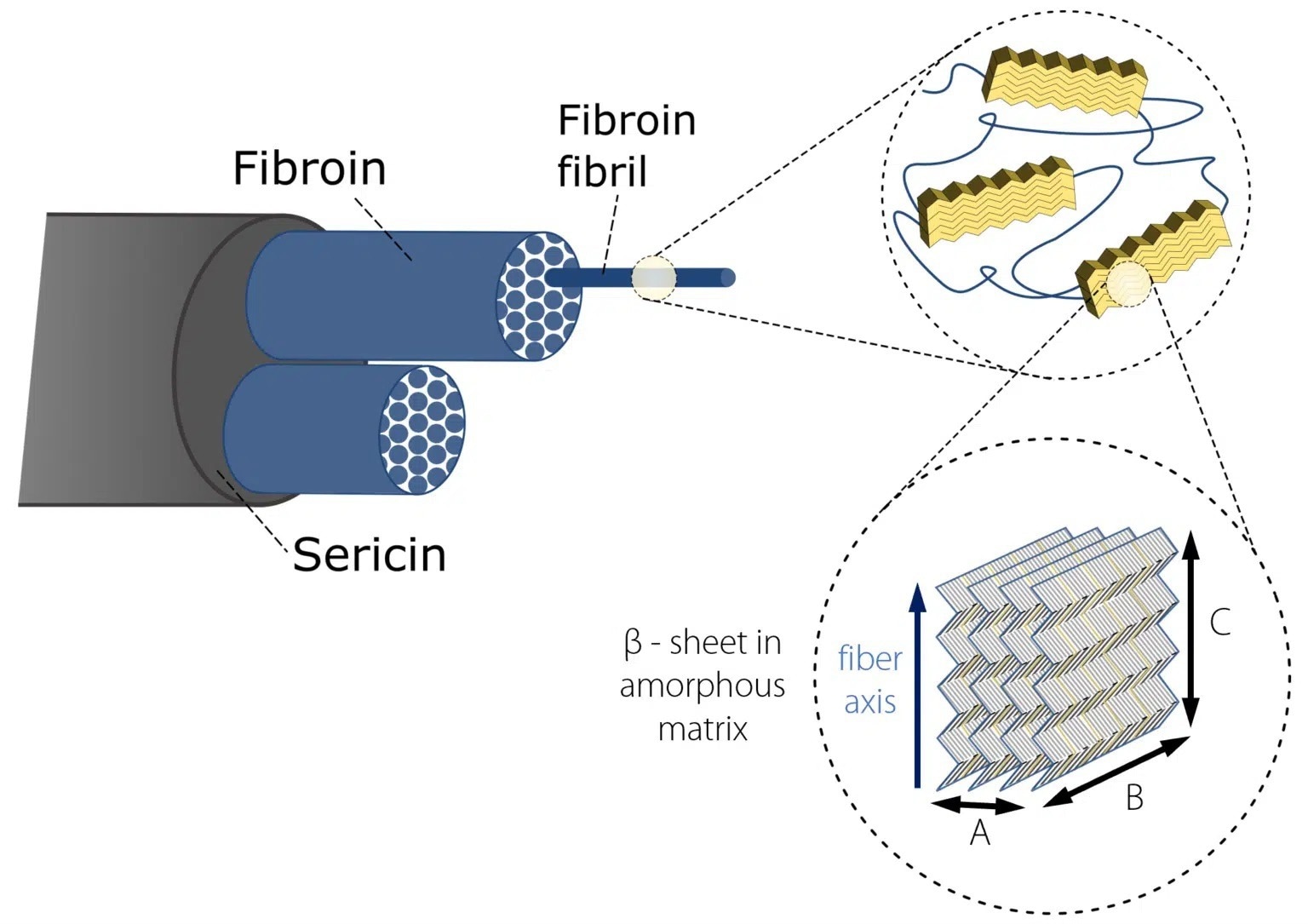
Figure 1. Hierarchical structure of Bombyx Mori silk. Image Credit: Representation inspired by Du et al. 2006; Du et al. 2011).
In this article, WAXS measurements, performed on thin single fibers (approximately 25 μm and 20 μm in the raw and degummed state, respectively, as measured by scanning electron microscopy (SEM)), have been used to study the effect of degumming on the crystalline structure of silk.
Click here to gain access to the complete application note
Acknowledgments
Xenocs recognizes Professor Erik Reimhult from the University of Natural Resources and Life Sciences, Vienna (Austria), and HRSM Nanobild for granting the team access to the scanning electron microscopy platform.
References
- Oduor E.O., Ciera L.W., and Kamalha E. “Applications of Silk in Biomedical and Healthcare Textiles.” Textiles for Functional Applications (2021) 75.
- Sobajo C., Behzad F., Yuan X.F., and Bayat A. “Silk: a potential medium for tissue engineering.” Eplasty 8 (2008).
- Du N., Liu, X.Y., Narayanan, J., Li, L., Lim, M.L.M. and Li, D., “Design of superior spider silk: from nanostructure to mechanical properties”. Biophysical journal, 91 (2006) 4528-4535.
- Du N., Yang, Z., Liu, X.Y., Li, Y. and Xu, H.Y., “Structural origin of the strain‐hardening of spider silk”, Advanced Functional Materials, 21 (2011) 772-778.

This information has been sourced, reviewed and adapted from materials provided by Xenocs.
For more information on this source, please visit Xenocs.