Fuel cells are becoming an increasingly popular alternative to combustion engines for clean power generation as countries and industries emphasize prioritizing the reduction of greenhouse gas emissions while fossil fuel markets remain volatile.
The global fuel cell market was valued at close to $4 billion in 2020, and current research has projected that it could reach $32 billion by 2030. The transportation and power sectors are expected to drive a significant growth percentage as both have been early to adopting fuel cells. This includes major automotive manufacturers such as Toyota and Hyundai, who manufacture fuel-cell-powered cars. Others, such as GM, are investing in fuel-cell solutions that could charge fully electric vehicles.

Image Credit: Ulbrich Stainless Steels & Special Metals, Inc.
Compared to traditional combustion power, the advantages fuel cell vehicles offer include improvements in efficiency, zero pollutant or greenhouse gas emissions, and an improved driving range on all-electric vehicles.
In the power sector, fuel cells supply energy for commercial, industrial, and residential buildings, as well as energy storage in the long-term for the grid in reversible systems.
As there is an increased demand for this innovative technology, the demand for advanced materials that allow fuel cells to reach peak performance will also grow.
What are Fuel Cells?
Fuel cells are electrochemical cells that produce electricity using only chemical fuel and an oxidizing agent. Fuel cells that rely on hydrogen as chemical fuel are completely carbon-free – their only byproducts are electricity, heat, and water.
Fuel cells can vary considerably in size and applied technologies based on the end application. Some fuel cells are smaller, similar to conventional cell types, and used to power forklift trucks. Others are large towers that could replace existing energy sources and potentially generate electricity (or backup power generation) for an entire facility.
How Do Fuel Cells Generate Electricity?
A fuel cell is predominantly comprised of two bipolar (anode and cathode) plates separated by a catalyst and electrolyte material.
In a hydrogen fuel cell, hydrogen gas flows into the negatively charged anode plate, while oxygen fills the positively charged cathode plate.
The hydrogen in the anode and the oxygen in the cathode wants to react and form water, but hydrogen cannot pass through to the oxygen side of the fuel cell because of the electrolyte. Electrolyte materials only allow positively charged ions through (hydrogen atoms are neutral with one proton and one electron).
However, a catalyst splits the hydrogen atoms apart, separating the electrons from the protons. This enables protons to pass through the electrolyte, with the electrons left behind.
To allow the electrons to access the protons and form water, a wire is connected to help the electrons flow through and react. As the electrons travel through the wire, water is produced that then drains from the system.
Electricity simply moves electrons, so the electrical current passing through that wire can power vehicles, facilities, or even satellites.
Fuel cells will always have the capacity to produce electricity while there is an available supply of fuel (in this case, hydrogen) and oxygen.
What Materials are Used to Manufacture Fuel Cells?
Fuel cell technologies can significantly vary depending on the application and power output needed. A general guideline is that the more power required, the larger the fuel cell must be.
This articles focuses on polymer electrolyte membrane (PEM) fuel cells, a common fuel cell type used for powering vehicles.
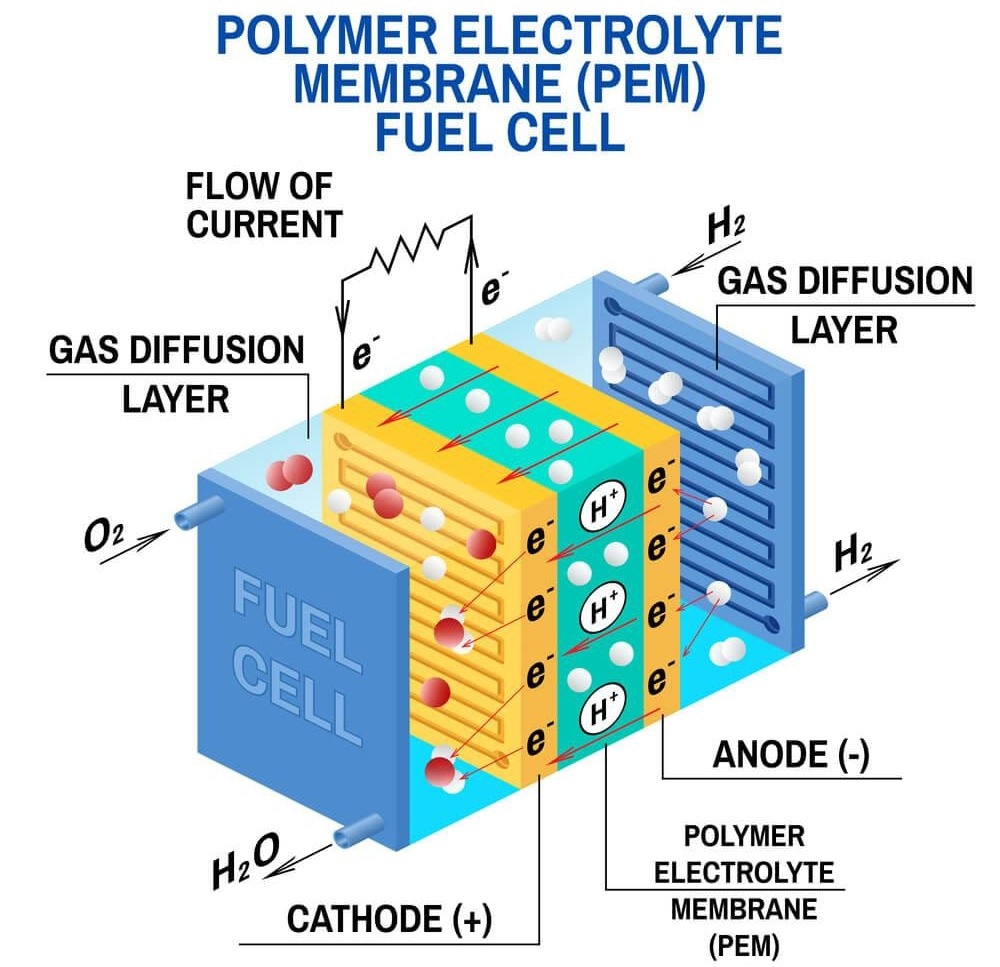
Image Credit: Ulbrich Stainless Steels & Special Metals, Inc.
The two primary components of a PEM fuel cell are the membrane electrode assembly (MEA) and the hardware surrounding it.
The MEA includes the electrolyte membrane, the catalyst layers, and the gas diffusion layers, where the chemical reactions take place to generate electricity.
Materials usually found in the MEA include polymers, ionomers, platinum powder (as the catalyst), and coated carbon paper (to diffuse gas and avoid rapid water build-up).
The hardware surrounding the MEA includes the bipolar plates and gaskets. Each individual MEA produces no more than 1 V, so generally, they are stacked to achieve the voltage required, with each cell divided by bipolar plates and gaskets to maintain a tight seal.
These bipolar plates are the most common fuel cell component manufactured with Ulbrich’s precision alloys, but other assorted metal parts are also available.
What are the Key Material Properties Needed to Optimize Fuel Cell Applications?
Fuel cells get extremely hot when in operation, and their environments are highly corrosive. The material durability under these challenging conditions is crucial for this market.
Austenitic stainless-steel alloys, which are made up of at least 8% nickel and are resistant to extreme temperatures and corrosion, are typically used in fuel cell applications. Secondary processing by parts fabricators is common to give greater corrosion resistance to alloy fuel cell parts.
Corrosion and temperature-resistant special metals, such as Nickel 200 & 201, are also frequently used in fuel cell designs.
Alloys:
Alloy thickness is another key component. More power-generating fuel cell membranes can be stacked if the bipolar plates are thinner, producing a more effective system that can meet higher voltage needs.
As these alloy plates are stacked, tight tolerances and flatness are essential to ensure the system operates at optimal performance. The slightest inconsistencies are magnified exponentially with each stack, and if precision is not there, the fuel cell stack may lean. Tight tolerances are also vital for the secondary processing completed by fuel cell parts manufacturers.
Extra attention is given to how distinctive surface finishes will influence the reliability and efficiency of the fuel cell as different applications and various fuel cell technologies demand different bi-polar plate finishes.
The impact surface finishes can have on corrosion resistance alone signifies how important it is to source fuel cell material from suppliers with state-of-the-art surface control capabilities.
Precision re-rollers offer greater levels of control over surface finishes compared to sourcing directly from a melt mill. For instance, Ulbrich works with its customers to offer a variety of custom application and process-specific finishes that are also specialized for the task at hand.
Generally measured in RA, RY, and RZ (evaluation of different aspects of the peaks and valleys of a surface), precision is essential to ensure the numbers remain consistent throughout the material’s length and width. This helps create a uniform and consistent surface finish that offers engineers and fuel cell manufacturers predictable performance reliability.
While each fuel application varies, there are different considerations that must be taken into account for each alloy used. Gas diffusion, longevity, electrical conductivity, and other vital performance factors can be impacted by the temper, finish, and gauge of all alloys used.

Image Credit: Ulbrich Stainless Steels & Special Metals, Inc.
What Should I Look for When Choosing a Fuel Cell Materials Supplier?
Capability and availability are of utmost importance in the fuel cell market.
Fuel cell applications can differ wildly, therefore, a precision re-roller with the technical capabilities and metallurgical expertise to match alloy solutions to the particular needs of your application is required. If necessary, they should be fitted to assist technical teams with research and development as various properties and material variables are being tested.
When it comes to accessibility, selecting a material supplier that can provide an extensive range of readily available alloys will help ensure that an optimal grade is available for a given application.
At Ulbrich, close technical collaboration between metallurgists and product development teams sets out the path for success. Once optimal material characteristics have been determined, Ulbrich’s tight tolerance control, extensive range of finishes, large alloy selection, and effective manufacturing processes lead to consistent production of custom-built products for the specific needs of each different fuel cell application.
Ulbrich’s service center distribution hubs enable the company to augment its precision re-roll capabilities and deliver a consistent supply of material to fuel cell customers, including wide-width and light-gauge products from Ulbrich’s California location.
If you are a fuel cell manufacturer or a parts fabricator, Contact Ulbrich to learn how advanced fuel cell materials expertise and manufacturing capabilities can support your application.

This information has been sourced, reviewed and adapted from materials provided by Ulbrich Stainless Steels & Special Metals, Inc.
For more information on this source, please visit Ulbrich Stainless Steels & Special Metals, Inc.