According to a recent study by King Abdullah University of Science and Technology (KAUST), rechargeable batteries that utilize ammonium cations (NH4+) as charge carriers could offer environmentally benign and long-lasting alternatives to metal-ion-based batteries.

Image Credit: Black_Kira/Shutterstock.com
Metal-Ion Batteries
The most preferred form of energy storage is metal-ion batteries, like lithium-ion batteries. Because of their great energy density and adaptability, they dominate the markets for portable consumer electronics and electric cars. However, the long-term supply of the metal ions utilized in the electrolytes is threatened by their reliance on finite and depleting resources. They can also be environmentally hazardous and unsafe due to their toxicity and flammability.
Existing Limitations of Ammonium Cations
Because ammonium cations are light and simple to synthesize and recycle, there have been numerous attempts to produce ammonium-ion-based batteries to address sustainability and environmental challenges. However, at low operating potential, ammonium cations are vulnerable to reduction into hydrogen and ammonia, which prevents the batteries from operating to their full capacity. They are also challenging to include in electrode materials since they easily dissolve in solutions.
New Research on Metal-Free Batteries
Because of their substantial reliance on nonrenewable metals, popular metal-ion batteries (MIBs) cause economic and environmental problems. In an attempt to solve this problem, Husam Alshareef, Zhiming Zhao, and their colleagues combined an ammonium-cation-containing electrolyte and carbon-based electrodes to create a high-efficiency, metal-free battery in their recent study published in the journal Angewandte Chemie.
In this study, they discussed the development of a dual-ion battery based on an ammonium compound. The proposed battery had a record-breaking 2.75 V operating voltage. The authors observed the reversible anion (PF6) intercalation chemistry in the graphite cathode and the NH4+ intercalation behavior in the PTCDI (3,4,9,10-perylene tetracarboxylic diimide) anode was responsible for the operating mechanism of the proposed environmentally-friendly battery.
The researchers found that the decreased susceptibility of NH4+ and the unavailability of mature NH4+-rich cathodes for NH4+ ion batteries were successfully overcome by the innovative battery architecture. The graphite||PTCDI complete battery had a high energy density of 200 Wh kg-1 and a long-lasting lifespan of over 1000 cycles, thanks to the specialized organic NH4+ electrolyte. It was demonstrated that to avoid NH4+ reduction, the development of ammonium-ion batteries should be high-voltage focused while avoiding low operation potential.
According to Zhao, the organic semiconductor anode and the graphite cathode are inexpensive, sustainable, and regenerative. The team developed a dual-ion battery by using hexafluorophosphate ions as negative charge carriers for the ammonium cations and making use of graphite's capacity to accommodate these anions inside its layers reversibly.
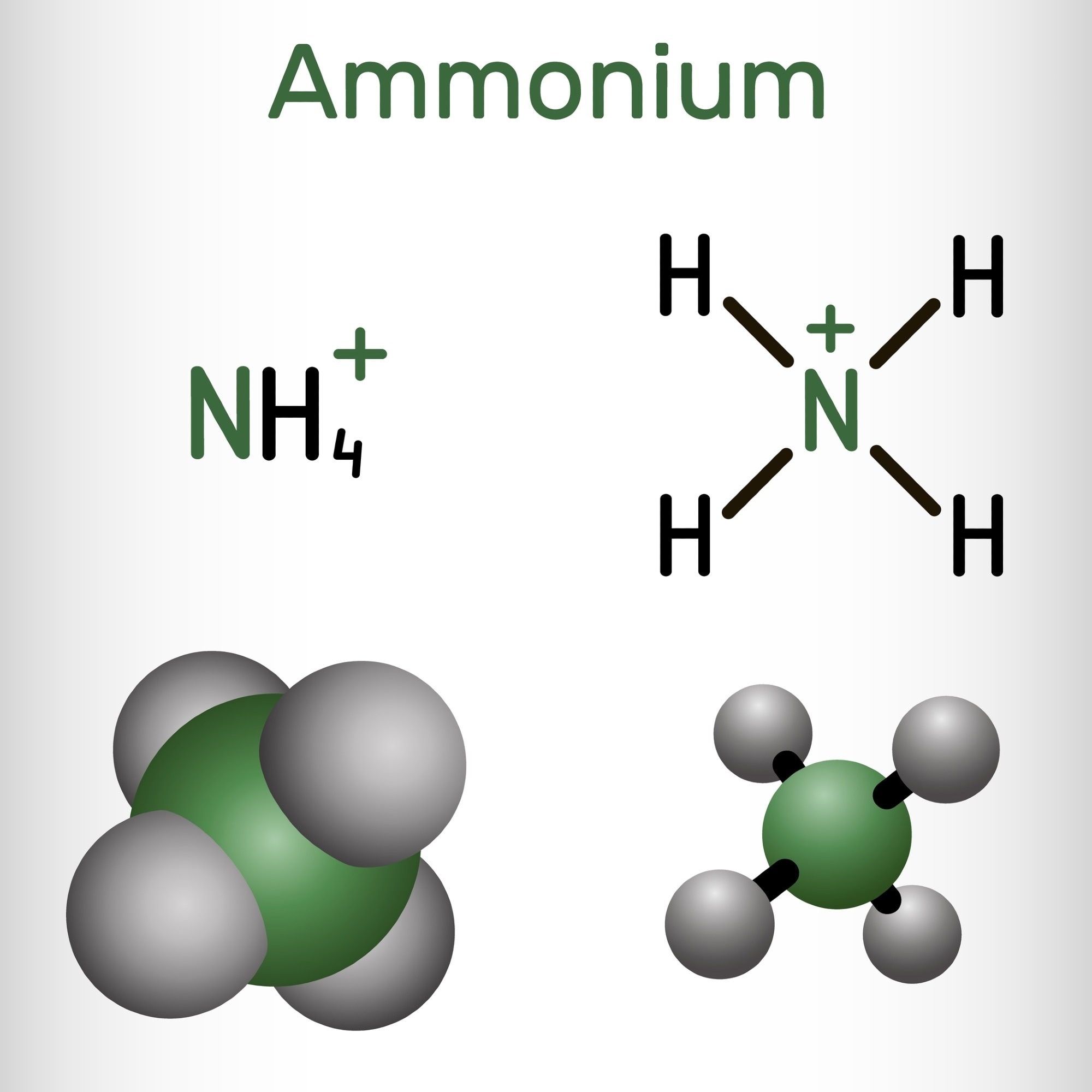
Image Credit: Bacsica/Shutterstock.com
When the battery is charging, cations and anions are simultaneously inserted into the appropriate electrode, and when the battery is discharging, they are released into the electrolyte. This sets their research apart from other studies, according to Zhao.
He explained that by screening a series of solvents resistant to high voltage and also taking into account its reduction stability, an electrolyte could be produced that is both antioxidative and anti-reductive. While its anti-reductive counterpart created a solvation sphere around the cations involved in the anode reaction, the antioxidative solvent mostly solvated anions engaging in the cathode reaction.
Zhao further explained that the proposed setup is essential for battery stability. With a high operating voltage of 2.75 V, the battery beats ammonium-ion-based counterparts already on the market. He asserted that it is now feasible to create high-energy nonmetallic ion batteries that are competitive with metal-ion batteries.
In order to move closer to large-scale applications, the team is actively striving to improve performance. They are also investigating anode materials with a higher capacity to increase energy density.
Lithium-Ion Battery Substitutes
A team led by Husam Alshareef from KAUST is creating inexpensive lithium-ion battery substitutes, especially for grid-scale storage. According to the team, battery costs must considerably decrease for the grid to eventually be decarbonized. These expenses can be reduced by using nonmetallic charge carriers in place of lithium, such as ammonium ions.
Furthermore, the University of Southern California (USC) is working on a grid-scale flow battery that is water-based, metal-free, and can be more quickly and cheaply manufactured than conventional batteries. Instead of storing energy within the battery container, external tanks are used to store chemical energy in flow batteries.
This makes it possible to scale up at a reasonable cost because increasing storage capacity only requires expanding the tank. Batteries for grid-scale energy storage must be affordable, reliable, and sustainable — requirements that many of today's established battery technologies fall short of.
USC's novel flow battery has the potential to lower costs, boost durability, and store higher amounts of excess energy, supporting the broader deployment of renewable energy sources. It does this by utilizing cutting-edge designs and incredibly affordable organic materials.
References and Further Reading
News Release, Metal-free batteries raise hope for more sustainable and economical grids, EurekAlert, January 10, 2023. https://www.eurekalert.org/news-releases/976130 Source: https://discovery.kaust.edu.sa/en
Zhao, Z., Lei, Y., Shi, L., et. al., A 2.75 V ammonium-based dual-ion battery, Angewandte Chemie, 61 (51) e202212941 (2022). https://onlinelibrary.wiley.com/doi/10.1002/anie.202212941
Inexpensive, Metal-free, Organic Flow Battery, University of Southern California (USC). Source - https://arpa-e.energy.gov/technologies/projects/inexpensive-metal-free-organic-flow-battery
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.