Sponsored by m-oemReviewed by Olivia FrostMay 10 2023
Paints and coatings add color to our lives, making products and materials safer and easier to use. The steady growth in annual production reflects the importance of this industry.

Image Credit: ShutterStock/Sebastian Duda
From 2007 to 2012, the annual global compound growth rate of the coating industry in GDP was 4.3%, with a revenue of 110 billion USD for 2012.1 Growth has since been on the rise due to the demand from the Asia Pacific, Eastern Europe, and Latin America regions.2
The primary reason for the importance of paints and coatings comes down to the range of application potential. They are applied to make substrates look nicer and for protection, insulation, anti-skid surfaces, or as electrical conductors.
To meet the application’s demands, complex formulations, which are made of solvents, binders, pigments, and additives, are produced. The individual amounts of these may vary significantly. For example, solvents are unnecessary when developing powder coatings (see Figure 1).
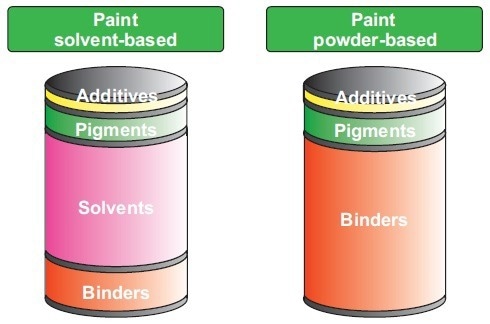
Figure 1. Overview of ingredients and possible amounts of solvent-based and powder-based coatings. Image Credit: B&W Tek
Users expect products that meet their expectations and application requirements, which can be achieved through the smart combination of these main components. Quality control procedures must be reliable and fast-performing to guarantee optimal results. However, this becomes increasingly difficult as the complexity of products increases.
Quality Control – Essential at Every Step
Quality control must be conducted throughout manufacturing to ensure the end product is up to standard. The individual production steps have various requirements regarding the analysis. Three main production steps with varying analysis requirements are listed below:
High Throughput is Key in the Warehouse
In the warehouse, it is crucial to identify and validate the incoming raw materials for paint and ink production for quality and purity. Analytical methods that can replace time-consuming wet chemical procedures should be used to meet high throughput demands.
Precision and Accuracy for Successful Production
Throughout each step of formulation and production, the quantity of various characteristic values is set and the identity of the intermediates is evaluated. These control mechanisms are conducted at line (next to the process) and online (in a process bypass).
Precision and accuracy parameters are crucial in each one of these steps. The appropriate analytical method should be selected with care and attention to detail.
Complex Matrices Demand Powerful Methods
Before delivery, the last and crucial step in quality control is the final testing. All general quality and relevant properties must be validated to assure optimal product performance at the user end. As the variety of components of the end product means complex matrices, final testing can quickly become an arduous task. Therefore, combining a range of suitable analytical instruments is critical to obtaining reliable results.
Many Parameters – A Challenging Task
Manufacturers have access to instrumental and wet chemical methods, which are used throughout the entire production process. For reference, the standard operating procedures as set out in the ASTM methods can be applied (see Table 1).3
Table 1. Overview of ASTM Methods for key parameters and the corresponding methods and instruments. Source: B&W Tek
| Parameter |
Instrument/method |
ASTM Method |
| Density of liquids |
Pycnometer |
ASTM D1475 |
| Water in paint |
Titration |
ASTM D4017 |
| Particle size |
Oil adsorption of a pigment |
ASTM D1483 |
| VOC content |
Chromatography |
ASTM D6886 |
| Powder Coating |
Colorimetry |
ASTM D3451 |
| Viscosity |
Rotational viscometers |
ASTM D2196 |
This section alone demonstrates why various analytical instruments and methods are required, which results in a significant training effort for employees, which costs time and money.
Wet Chemical Analysis – A Long Tradition
Traditional wet chemical analysis methods include titration, ion chromatography, and colorimetry. Each is highly accurate with excellent precision, and due to their long-standing importance as analytical tools, the research and literature regarding performance is well documented. However, these techniques are still limited to specific parameters. Additional costs stack up for purchasing auxiliary chemicals required for sample preparation and analysis.
Implementing strict safety regulations and training employees regularly adds to the costs. Therefore, finding and accessing alternative instrumental methods has been gaining more attention.
Specific instrumental methods can be conducted with minor training processes while fast and requiring fewer auxiliary chemicals. These benefits can be seen when using Vis-NIR spectroscopy.
Vis-NIR Spectroscopy – A Simple Solution
The visible-near-infrared (Vis-NIR) technology is a well-established analytical method used in the chemical, pharmaceutical, food, and feed industries. Its exceptional accuracy and reliability have given rise to expansion across various application fields.
The flexibility of Vis-NIR spectroscopy, seen in its versatility during process analysis as an inline, online, outline, and offline tool, has also contributed to its rising popularity as an analytical method.
The Functionality of Vis-NIR Spectroscopy
The measurement principle that underpins Vis-NIR spectroscopy is predicated on a non-destructive interaction between light and matter. This interaction is applied to identify several physical and chemical parameters within one measurement, which generally only takes a few seconds.
The combination of visible and near-infrared light enables users to acquire information relative to the color of the sample as well as information associated with its chemical side groups.
Economical and Ecological
Vis-NIR spectroscopy can achieve high throughput by identifying several parameters in a single measurement. Moreover, it meets all economic and ecological criteria.
The need for expensive auxiliary chemicals is eliminated as sample preparation is not required for liquid or solid samples, and the analyses are non-destructive. At the same time, safety is boosted and costs are significantly reduced.
Vis-NIR spectroscopy as a non-destructive, ready-to-use technique with great potential, particularly for the growing field of powder coatings that better complies with volatile organic compounds (VOC) regulations.
Pigments
Improving the aesthetics of a substrate is the most obvious application of paints and coatings. With a concentration range of 0–30%, pigments comprise some of the central ingredients in most paints and coatings. In addition to the intensity of the color, pigments also determine the opacity, coating thickness, and the coating’s gloss. Each of the properties is determined by the chemical and physical structure of the pigments. These are typically grouped as the crystallinity, particle size, and particle distribution.
DIN 5943 categorizes pigments relative to their chemical structures and their optical effects. The first classification sorts pigments into inorganic and organic pigments. Subsequently, they are then grouped into white, colored, effect, and luminous pigments.
To acquire the requisite properties in the final product, it is crucial that both the appropriate pigments are selected, as well as ensuring the pigment concentration is correct. Coating layer thickness and color intensity result from total pigment volume concentration (PVC), relative to the volume of pigments to the volume of all solids.3
In addition to the PVC, the binder/pigment concentration relationship is crucial. This parameter is critical pigment volume concentration (CPVC) and must be met as a shortfall harms paint stability and strength.3 Therefore, validating the pigment concentration is key. Analysis by Vis-NIR spectroscopy is described in the following section.

Image Credit: B&W Tek
Quantification of Dye Content in Inks
Triphenylmethanphenazin and azo dyes are traditionally used for ball-point pen inks. The inks are comprised of several ingredients, including the dye itself. Some of these additional ingredients include solvents to avoid clogging and surfactants to prevent foaming.
Establishing the absolute dye content can be a challenge due to the complex matrix of the ink components. In application note AN-NIR-026, 20 ink samples were evaluated with respect to the concentration of blue ink dye in ball pens.4
The concentration range examined was between 0.56% and 4.56%. Figure 2a shows a selection of the spectra. The data obtained during the evaluation were associated with the values from the primary method, which resulted in a high correlation, as illustrated in Figure 2b.
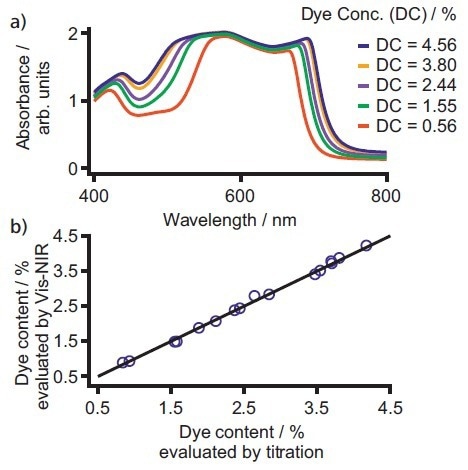
Figure 2. a) Exemplary spectra for five different dye concentrations typically used for ball pen inks. The visible region 400–800 nm displays a high correlation between the dye quantity and the absorbance of the sample. b) Correlation plot of dye content determined by Vis-NIR spectroscopy and titration. Besides the high correlation of R2 = 0.99, the standard error of validation was < 0.1% with two factors used. Image Credit: B&W Tek
Additives
Although the overall amount of additives makes up for a relatively small percentage of paint and coating formulations, they are crucial for modifying certain properties for specific applications.
Besides these product-specific additives, which are only observable in small concentrations, three primary additives are usually present in all paint and ink formulations: paint driers, surfactants, and antioxidants.
These control critical properties of the final product, such as brushability, sprayability, drying time, durability, and processability. As small changes in the number of additives introduced into a formulation can significantly alter the behavior of the final product, several chemical and physical properties of each additive must adhere to high-quality standards.
Table 2 summarizes the standard parameters of paint driers and their limits as defined by ASTM. Vis-NIR spectroscopy is a valuable tool to aid quality control as a method that can be used to determine multiple parameters with just one measurement.
Table 2. Overview of relevant parameters and respective limits for quality control of paint driers according to ASTM.3 Source: B&W Tek
| Parameter |
Specification |
ASTM Method |
| Specific gravity / - |
±0.05 |
ASTM D1289-85 |
| Solid content / % |
±5.00 |
ASTM D1644 |
| Metal content / % |
±0.30 |
ASTM D2373-85 |
| Dynamic viscosity / mPas |
±1.00 |
ASTM D2196 |
Multiple Parameter Analysis of Paint Driers
Paint driers are a crucial element of every paint or coating product because they modify the drying process of paints and coatings.
The drying process can be separated into two phases: The first is the physical process, where the solvent evaporates. The second phase is the chemical process, where the binder experiences an oxidative crosslinking reaction.
Paint driers made up of metal ligands expedite the second phase, which means the coating finish is sometimes reduced from hours to minutes. However, the catalytic function is contingent on the total metal amount, where even minute differences between concentrations considerably alter the behavior of the formulation.5
As a result, it is necessary to ensure that the concentration is quantified accurately during production. The most frequently used paint driers are cobalt octoate complexes. Formulations made with these additives are evaluated with respect to the metal content, viscosity, and specific gravity, which can be easily assessed within one measurement using Vis-NIR spectroscopy as shown in AN-NIR-033 (see Figure 3).6
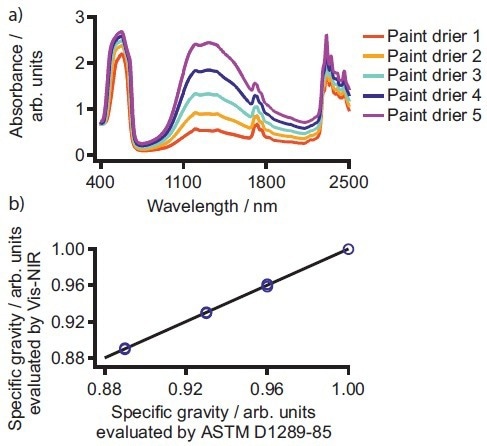
Figure 3. a) Exemplary spectra for different cobalt octoate paint driers. b) Correlation plot of values for the physical parameter viscosity obtained according to ASTM D1289-85 and measured by Vis-NIR spectroscopy. Image Credit: B&W Tek
Binders
The concentration ratio of binders and pigments (CPVC) can have a considerable impact on the stability of the final coating. Binders help make a coating solid and long-lasting. During the drying process, binders undertake a chemical cross-linking reaction.
Ensuring the coating affixes onto the substrate is essential. Therefore, binders are considered the most crucial paint and coating product ingredient. Compared to pigments and solvents, they can be found in every coating and paint formulation.
The most commonly used binders are polymeric substances, which include polyurethanes, polyamides, polyester, or epoxy resins (see Figure 4). Synthetic resins have replaced the natural resins traditionally used in paint and coating formulations. The binder impacts the CPVC, which controls the hardness and adhesion of the final product, as well as the viscosity.5
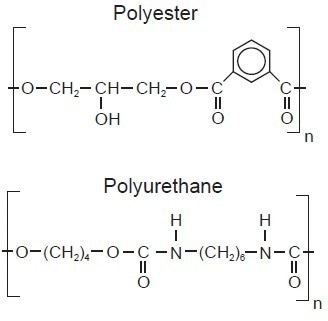
Figure 4. Chemical structures of two typical binders used in paints and coatings. Image Credit: B&W Tek
Free amines in qualitatively poor polyurethane binders can have a detrimental impact on certain coating procedures. The following section discusses how Vis-NIR spectroscopy can support the quality control process by identifying free amines in paints and coatings.
Quantification of Amine Value in Dipping Paints
A standard method used to apply uniform coatings is the electrodeposition process (EPC) for dipping paints. The primary benefit of this process is that the thickness of the layer can be manipulated with high precision even when confronted with more complex structures.
Because EPC is an electrochemical process, interfering with redox-active molecules can impede the desired coating result. These interfering molecules often arise due to the residuals of other coating constituents, e.g. free nitrogen as a residual impurity of the used binder.
Application Note AN-NIR-030 details how Vis-NIR spectroscopy can quantify the amine number as a gauge for free nitrogen in dipping paints.7 The concentration range was 33.82–48.31 mmol/kg, with a correlation of R2 = 0.97 with five PLS factors (see Figure 5).
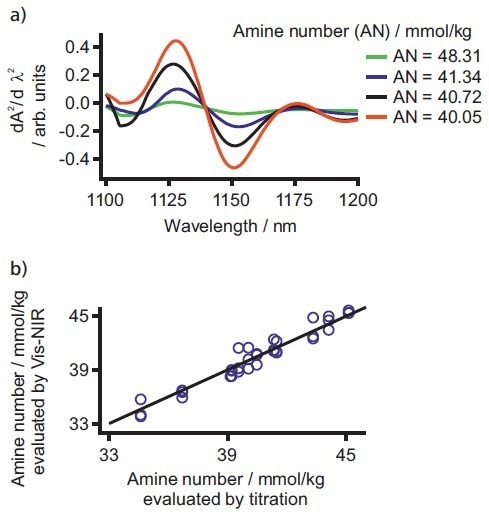
Figure 5. a) Exemplary spectra of the amine number analysis in dipping paints. b) Correlation plot of amine number values determined by Vis-NIR spectroscopy and titration. Image Credit: B&W Tek
Solvents
Solvent-based coatings, where the main element is the solvent, are the most popular class of coatings. The two main parameters of the solvent are the rate of evaporation and the solvating power, both of which impact the final product's viscosity and wetting behavior. The solvent also influences several aspects of product-specific behavior.
Therefore, carefully selecting products is paramount when considering the intended application. Solvents are typically categorized according to their chemical properties. Solvents can be categorized into three classes: hydrocarbon solvents, oxygenated solvents, and other solvents, e.g., water.3
The latter are found in all water-based coatings, gaining a more crucial role in recent years because they contain lower levels of volatile organic compounds (VOCs). There are restrictions on the use of VOCs in some countries and states. Water-based products are therefore becoming more prominent in the market.
Vis-NIR spectroscopy, as a precise technique, can help facilitate the quality control of solvents, particularly for water-based coatings.

Image Credit: B&W Tek
Analysis of Cosolvents in Water-based Paints
As previously highlighted, binders, pigments, and additives are vital ingredients in paint manufacture. To use these compounds in water-based coatings, so-called cosolvents must be introduced.
Cosolvents, such as butylglycol and propylheptyl alcohol, help solubilize binders and can be used for rheology control and as plasticizers. Plasticizers control several properties of coatings, such as dry film appearance or substrate adhesion.8
Metrohm Application Note AN-NIR-029 describes using Vis-NIR spectroscopy to quantify butylglycol and propylheptyl alcohol content in various water-based paints.9 Prediction models predicated on spectral data and reference values for each solvent facilitated a high prediction accuracy using Vis-NIR spectroscopy with a standard error of validation <1% (see Figure 6).
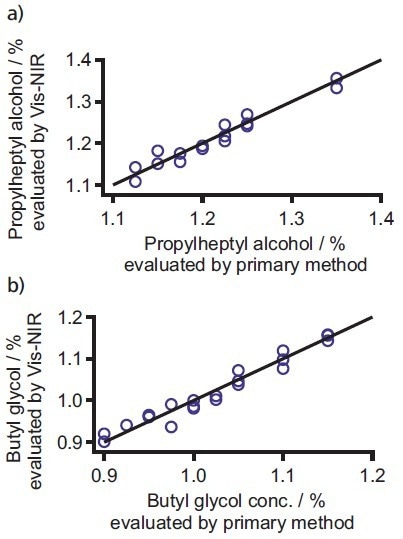
Figure 6. Correlation plots of a) propylheptyl alcohol and b) butyl glycol. Image Credit: B&W Tek
Summary and Conclusion
The application of paints and coatings is important across a wide range of purposes and, as a result must meet the high demands of user expectations.
These complex products must be subjected to robust quality control measures, necessitating sophisticated technologies to ensure reliable and accurate analysis. Needs are met by Vis-NIR spectroscopy, which fulfills its economical and ecological requirements.
Due to the fact Vis-NIR spectroscopy combines analysis of the visible spectral range and the near-infrared spectral users determine distinct chemical parameters, e.g. the amine number and concentrations of individual constituents, in addition to physical parameters, such as the particular density and dynamic viscosity.
The advantage that Vis-NIR spectroscopy offers in contrast to traditional methods, is that multiple parameters can be evaluated simultaneously using one measurement in under one minute without compromising the sample.
The applications outlined in this article offer insight into the possibilities that Vis-NIR spectroscopy offers. Because of its numerous advantages, Vis-NIR spectroscopy can complete and, in part, replace traditional methods used in the quality control of paints and coatings, which will make it an essential tool in the coming years.
References and Further Reading
- Scott Detiveaux, and Charles Bangert, (2016). The State of the Global Coatings Industry : Products Finishing. [online] Pfonline.com. Available at: https://www.pfonline.com/articles/the-state-of-the-global-coatings-industry [Accessed 28 Sep. 2016].
- Ihs.com. (2016). Paint and Coatings Industry Overview - Chemical Economics Handbook (CEH) | IHS Markit. [online] Available at: https://www.scribd.com/document/154065358/9453-0413PO-4746-0112PO-Chemical-Economics-Handbook-Lowres [Accessed 28 Sep. 2016].
- Koleske, J. (2012). Paint and coating testing manual. West Conshohocken, PA: ASTM International.
- Partners.metrohm.com. (2016). AN-NIR-026. [online] Available at: http://partners.metrohm.com/GetDocument?action=get_dms_document&docid=2353270 [Accessed 28 Sep. 2016].
- Gupta, A. (2013). Determination of optimal quantities of different types of driers for addition in the batches of paint formulation. International Journal of Engineering, Science and Technology, 5(4), pp.1-13.
- Partners.metrohm.com. (2016). AN-NIR-033. [online] Available at: https://www.metrohm.com/en_gb/applications/application-notes/nahinfrarotspektroskopieannir/an-nir-033.html [Accessed 28 Sep. 2016].
- Partners.metrohm.com. (2016). AN-NIR-030. [online] Available at: http://partners.metrohm.com/GetDocument?action=get_dms_document&docid=2405651 [Accessed 28 Sep. 2016].[Accessed 28 Sep. 2016]. [Accessed 28 Sep. 2016].
- Stoye, D. and Freitag, W. (1998). Paints, coatings, and solvents. Weinheim: Wiley-VCH
- Partners.metrohm.com. (2016). AN-NIR-029. [online] Available at: http://partners.metrohm.com/GetDocument?action=get_dms_document&docid=2404593 [Accessed 28 Sep. 2016].

This information has been sourced, reviewed and adapted from materials provided by B&W Tek .
For more information on this source, please visit B&W Tek .