Alumina (Al2O3) has excellent mechanical strength, hardness, thermal conductivity, and a high melting point and resistivity. As a result, it can be widely employed as wear-resistant material, polishing material, thermally conductive material, refractory material, and other materials.
Since it is a polar compound, the alumina particles often form aggregates due to the interaction of forces such as van der Waals and electrostatic forces. As a result, it is worth studying the stability of the alumina particle system with various surface modifications.
Zeta potential relies on the chemical composition of the particle surface and the medium environment. The particle system may display different zeta potentials at different pH.
This article presents an experiment using the BeNano analyzer and the BAT-1 autotitrator to obtain measurements of the zeta potential of alumina particles at different pH levels.
Principle
Electrophoretic light scattering (ELS) is employed to measure zeta potential, with the method involving a laser beam to irradiate the sample, with the scattered light being detected at a forward angle of 12 °.
The sample solution or suspension is exposed to an electric field applied to both ends of the sample cell. This results in the electrophoretic movement of the charged particles within the sample.
Subsequently, the frequency of the scattered light moves in comparison to the incident light, caused by the Doppler effect. This phase shift of scattered light signals is determined via PALS analysis.
The velocity of electrophoretic movement per unit electric field, denoted as the electrophoretic mobility µ, is obtained using the phase plot. Using Henry’s equation, the zeta potential ζ using the electrophoretic mobility µ. This can be calculated as follows:
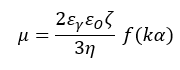
In this equation, ε0 represents the solvent dielectric constant in the vacuum and εγ represents the relative dielectric constant. The solvent viscosity is denoted by η, while the Henry function is denoted by f(Κα), where Κ is the reciprocal Debye length and α is the particle radius. This means that Κα is the ratio between the thickness of the double layer and the particle radius.
The BeNano 90 Zeta from Bettersize Instruments Ltd. measures zeta potential in this experiment.
Experimental
The Al2O3 powder was dispersed in pure water and stirred using a magnetic stirrer for 15 minutes. The initial pH was 6.4 with a positive zeta potential. The BAT-1 autotitrator was combined with the BeNano analyzer to conduct the NaOH titration automatically.
The tolerance and pH interval were set to 0.2 and 1, respectively, and the measurement temperature was 25 ℃ ± 0.1 ℃. A single measurement of zeta potential measurement was obtained at each target pH.
Results
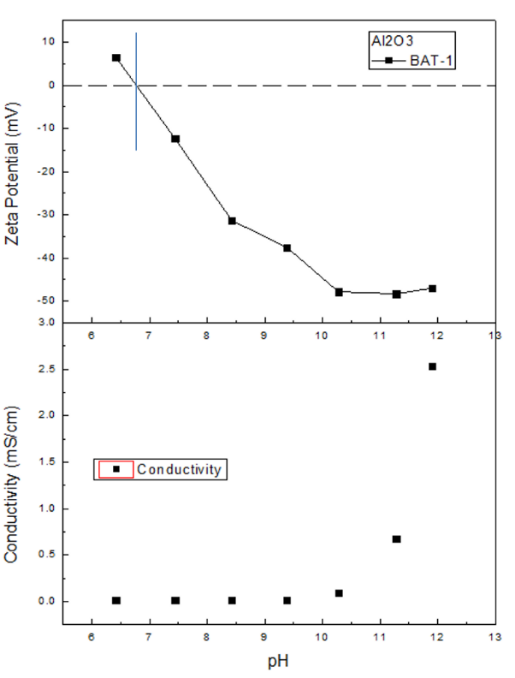
Figure 1. The zeta potential curve (above) and conductivity curve (below) at different pH. Image Credit: Bettersize Instruments Ltd
At the initial pH, the zeta potential of the sample is positive, as displayed in Figure 1. This suggests that the particles carried positive charges. As the pH level increases, the charges become negative because of the addition of the NaOH titrant. The dispersion reaches the isoelectric point (IEP) at a pH of 6.8.
Al2O3 is an amphoteric oxide, and the initial pH is almost neutral when dispersed in pure water. However, conducting the titration with a base alters the pH and neutralizes the positive charges of particle surfaces, and changes them to be negative.
In the higher pH range (when there are more negative charges on particle surfaces), there is a higher magnitude of zeta potential. This is indicative of the system being more stable and agglomerates being less likely to form. Near the IEP, the magnitude of zeta potential is the lowest, resulting in poor system stability.
When the pH exceeds 10, the negative charges are nearly saturated on the particle surface. Increasing the pH further cannot induce more negative charges on the surface, but adding NaOH increases the ionic strength.
Subsequently, the shielding effect is dominant and the magnitude of zeta potential is reduced.
Conclusion
The characterization of zeta potentials of Al2O3 at different pH was carried out using the BAT-1 autotitrator in combination with the BeNano analyzer. The results demonstrate that the IEP of the sample system is 6.8.
The magnitude of zeta potential close to the IEP is relatively low, indicative of an unstable sample system. At higher pH levels of 10~12, the magnitude of zeta potential is relatively high, increasing the stability of the sample system due to the stronger electrostatic force.
Acknowledgments
Produced from materials originally authored by Jing Cao and Hui Ning from the Application Research Lab at Bettersize Instruments Ltd.

This information has been sourced, reviewed and adapted from materials provided by Bettersize Instruments Ltd.
For more information on this source, please visit Bettersize Instruments Ltd.
