Sponsored by B&W TekReviewed by Olivia FrostJan 25 2024
The popularity of dietary supplements is rising, driven by advertising, consumer trust in “natural” products, and easy accessibility. Despite frequently being advertised as “alternative medicines,” dietary supplements are classified as food in the US and EU. Consequently, these products do not undergo the same testing, quality control, labeling, or regulation as pharmaceuticals.

Image Credit: B&W Tek
This incentivizes certain supplement manufacturers to exaggerate the effects of a supplement by illegal adulteration with undisclosed substances like analogs, pharmaceuticals, or unauthorized additives. While this approach delivers quick results and boosts sales, it could also result in health risks from side effects and acute intoxication.
For example, sport and sexual performance supplements often include undisclosed steroids, caffeine, hormones, and pharmaceuticals for the treatment of impotence. The resulting side effects of these additives can be dire and include heightened aggression, overdose, and heart attack.
Traditional analysis of dietary supplements is costly, time-consuming, and reliant on laboratory-based methods like GC-MS and HPLC. Quick on-site techniques are required for surveying the supplement landscape. Surface-enhanced Raman scattering (SERS) emerges as a solution to meet these demands. SERS is rapid, simple, highly sensitive, portable, inexpensive, and portable.
SERS uses nanoparticle substrates (which are available in various forms, such as colloids or printed materials) to enhance the Raman signal and generate a fingerprint spectrum of very low-level analytes.
Metrohm’s P-SERS substrates - convenient, single-use tests with nanoparticles printed onto paper strips - were employed in this study. P-SERS are suitable for quickly and cost-effectively identifying diverse trace materials.
Proof of Concept Studies
In a recent Heliyon publication, Professor Saskia van Ruth and her team at Wageningen University and Research conducted a comprehensive investigation into developing a SERS-based method to identify illegal contaminants in dietary supplements quickly.1
Professor Saskia van Ruth has dedicated almost 20 years to addressing food integrity issues as Professor of Food Supply Chain Integrity at University College Dublin (presently) and Professor of Food Authenticity & Integrity at Wageningen University and Research.
During her time at Wageningen Food Safety Research from 2005 to 2023, she performed high-quality, independent research into reliable and safe food. This involved measuring additives, toxins, and residues to detect risks, monitor trends, and uncover fraud in the food chain.
Professor van Ruth’s team conducted SERS investigations, aiming to develop a qualitative screening method for the identification of illicit adulterants in dietary supplements.
Qualitative Screening and Detection of Illicit Adulterants
Twenty-three pharmaceutically active adulterants were evaluated for SERS activity. Eleven of these analytes exhibited strong good signals when tested with Silver P-SERS substrates and the Metrohm Instant SERS Analyzer (MISA).

Image Credit: B&W Tek
The spectra of pure reference samples were gathered into a custom reference library, which was utilized for screening experimental samples. Table 1 lists the active analytes and their status in dietary supplement formulations as determined by the European Commission (EC).
Table 1. SERS-active adulterants, their type and current EC status in dietary supplement formulations as established by the European Commission, and a summary of results including Hit Quality Index (HQI) indicating the level of correlation between sample and library spectra and Limit of Identification (LOI) for each adulterant. Source: B&W Tek
| RESULTS |
|
|
|
| |
Supplement Type |
EC Status |
HQI |
LOI (% w/w) |
| Acetildenafil |
Erectile Disfunction |
Forbidden |
0.79-0.99 |
0.1 |
| Fluoxetine HCl |
Weight Loss |
Forbidden |
0.71-0.83 |
5.0 |
| Homo sildenafil |
Erectile Disfunction |
Forbidden |
0.79-0.99 |
0.1 |
| Melatonin |
Sleep Regulation |
Allowed |
0.79-0.93 |
5.0 |
| Phenethylamine |
Energy |
Forbidden |
0.81-0.94 |
0.5 |
| Piperin |
Weight Loss |
Allowed |
0.70-0.92 |
2.5 |
| Sildenafil Citrate |
Erectile Disfunction |
Forbidden |
0.79-0.99 |
0.1 |
| Synephrine |
Weight Loss |
Allowed |
0.70-0.81 |
5.0 |
| Thiosildenafil |
Erectile Disfunction |
Forbidden |
0.60-0.99 |
0.1 |
| Vardenafil HCl |
Erectile Disfunction |
Forbidden |
0.81-0.95 |
0.1 |
| Vinpocetine |
Memory Support |
Allowed |
0.61-0.98 |
0.5 |
Evaluation of Interference from Excipients
SERS’s distinctive insensitivity towards numerous excipients is a vital benefit of this method. A mixture of common excipients was meticulously formulated to mimic the filling material utilized in market supplements.
This formulation comprised titanium oxide (0.5 % w/w), hydroxypropyl methylcellulose (0.5 % w/w), silicon dioxide (1.0 % w/w), magnesium stearate (3.0 % w/w), and microcrystalline cellulose (95.0% w/w).
Each of these components was analyzed for SERS activity. It was then confirmed that they exhibited low/no SERS activity and were deemed unlikely to interfere with identifying the target adulterants.
Determination of LOI
To ascertain the detectability range of each adulterant, various concentrations were mixed with the excipient mixture (0.1–50.0 % w/w).
The artificially adulterated samples were processed as follows: 10 mg of every sample was weighed into an Eppendorf tube and diluted with 100 μL of EtOH. Every sample was then vortexed and centrifugated. 10 μL of the supernatant was subsequently applied onto the Silver P-SERS strip, dried for 15 minutes, and evaluated.
Simulation of Dietary Supplement Adulteration
Eighteen commercial supplements, including nine sexual and sport enhancement supplements and nine weight-loss and lifestyle supplements, were procured, tested for SERS activity, and adulterated within the 0.1 % to 5.0 % w/w range (shown in Table 1).
Ethanol was selected as a common, non-toxic solvent to extract the target without dissolving the matrix. Each sample was shaken in a small vial with ethanol. The supernatant was then added directly to a P-SERS strip. Following drying, instrumental analysis began.
MISA Cal software delivers a guided workflow, yielding results within seconds, accompanied by a Hit Quality Index (HQI) indicating the amount of correlation between the library spectra and sample. The Limit of Identification (LOI) for each adulterant is determined based on the lowest concentration at which the HQI exceeds 0.70.
Results
Under actual conditions of adulteration, this screening technique demonstrated high efficacy in identifying all the target sport and sexual enhancement adulterants (Acetildenafil, Homo sildenafil, Sildenafil Citrate, Thiosildenafil, and Vardenafil HCl). This method yielded high match scores (0.79–0.99), even at the lowest concentration level tested (LOI of 0.1 % w/w).
This suggests the successful identification of well-known pharmaceutical products on the market (such as Viagra® for sildenafil and Levitra® for vardenafil), as well as their unauthorized analogs well below active therapeutic doses.
For the lifestyle and weight-loss analytes, the screening method was able to identify Piperin with an LOI of 2.5 % w/w, Vinpocetine and Phenethylamine with an LOI of 0.5 % w/w, and Melatonin, Fluoxetine HCl, and Synephrine with an LOI of 5.0 % w/w.
Real World Application
Honey-Based Sexual Supplements
“Hot Honey” or “Honey for Men” is a readily available dietary supplement that promises “power and vitality.” However, the FDA cautions that such honey products may harbor undisclosed drugs for erectile dysfunction, such as tadalafil and sildenafil, in active concentrations.2
Metrohm Nederland utilized MISA to examine seized honey samples and demonstrate the efficacy of Metrohm’s SERS solutions in the combat against illegal adulteration.
An integral aspect of this application involves testing numerous SERS substrates to optimize the target signal. While Professor Saskia’s team predominantly focused on Silver P-SERS strips due to their convenience, the Metrohm group also assessed Gold P-SERS strips, along with Gold and Silver Colloid solutions.
Sample Preparation
Four honey samples were supplied, each with known concentrations of tadalafil sildafenil confirmed by GC-MS, as highlighted in Table 2.
Table 2. Seized samples of honey, laboratory tested and shown to have undeclared amounts of sildenafil and tadalafil, known erectile dysfunction drugs. Source: B&W Tek
| Sample |
Analyte |
Concentration |
| 1 |
Sildenafil |
20-50 mg/15 g |
| 2 |
Sildenafil |
20-50 mg/15 g |
| 3 |
Tadalafil |
50 mg/10 g |
| 4 |
Tadalafil |
20-50 mg/15 g |
To prepare the samples for SERS examination, a segment of each sample was dissolved into 1mM sodium hydroxide and subsequently shaken with sodium chloride and isopropyl alcohol (IPA). Following separation, the organic fraction was directly applied to P-SERS and colloid SERS substrates, as illustrated in Figure 1.
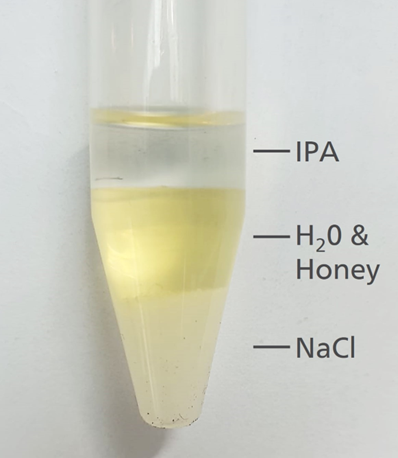
Figure 1. A simple solvent extraction procedure proved effective at moving target substances into an organic layer (IPA) in order to isolate them from the honey matrix. Image Credit: B&W Tek
Results
Sildenafil
Honey samples 1 and 2 were easily evaluated with Silver (Ag) P-SERS strips and Gold (Au) Nanoparticle colloid solution (NP). This is characteristic of substances that exhibit strong Raman activity and possess good binding affinity with SERS substrates. Figure to displays the spectra of samples 1 and 2 in comparison with reference spectra of Sildenafil from Metrohm.
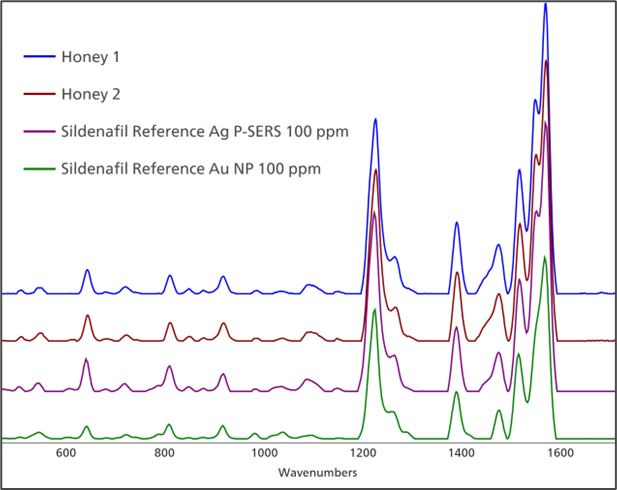
Figure 2. Both honey samples 1 and 2 compare favorably with SERS reference spectra of Sildenafil. Image Credit: B&W Tek
The honey itself does not significantly contribute to the spectrum, even with minimal sample preparation. The similarities are evident, and MISA Cal software correctly matches the substance to three different reference spectra of Sildenafil with extremely high Hit Quality Index scores (shown in Figure 3).
The evidence supporting sildenafil contamination is particularly robust as not only does the honey sample match within a custom SERS library, but its spectrum also matches with Sildenafil from Metrohm’s larger Illicit Library.
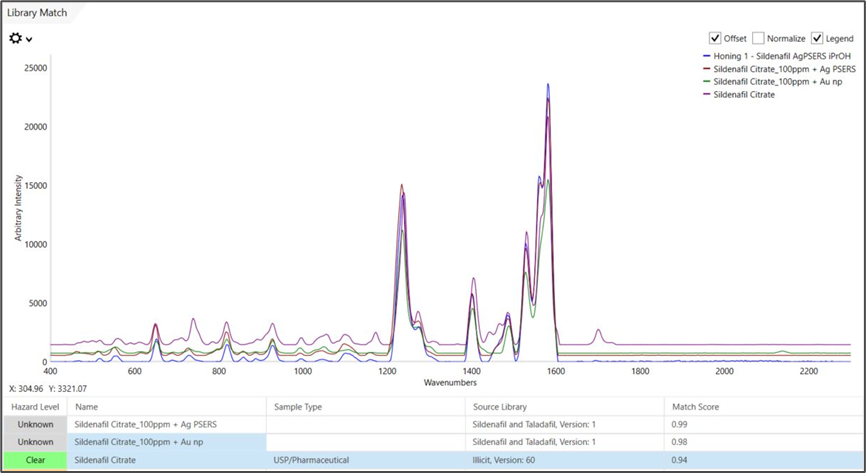
Figure 3. Library matches between the Honey 1 sample and Ag P-SERS and Au NP SERS spectra, in addition to a sildenafil spectrum from Metrohm’s Illicit Library. Note the very high HQI values achieved for all sildenafil matches (HQI values closest to 1 indicate very strong correlation of sample spectrum with library spectra). Image Credit: B&W Tek
Tadalafil
Both Silver and Gold P-SERS substrates delivered weak SERS enhancement for tadalafil. Thus, the Gold Nanoparticle colloid solution was tested and provided a good SERS signal, as shown in Figure 4.
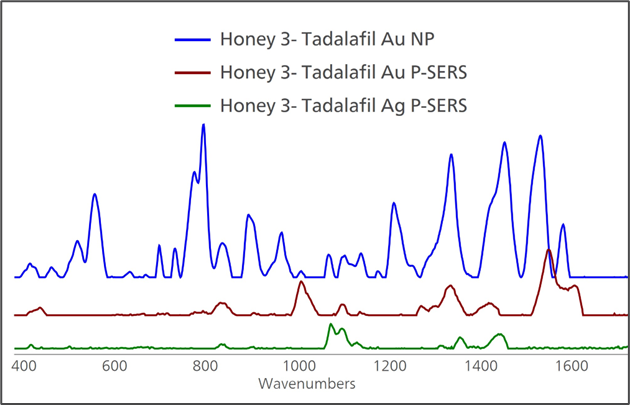
Figure 4. Out of three SERS substrates tested, including Au NP, Au P-SERS and Ag P-SERS, only Au NP solution provided a good SERS spectrum of tadalafil. Image Credit: B&W Tek
When Gold NP solution was utilized to assess samples 3 and 4, both compared favorably with a tadalafil reference spectrum. Thus, both 3 and 4 were accurately identified as tadalafil.
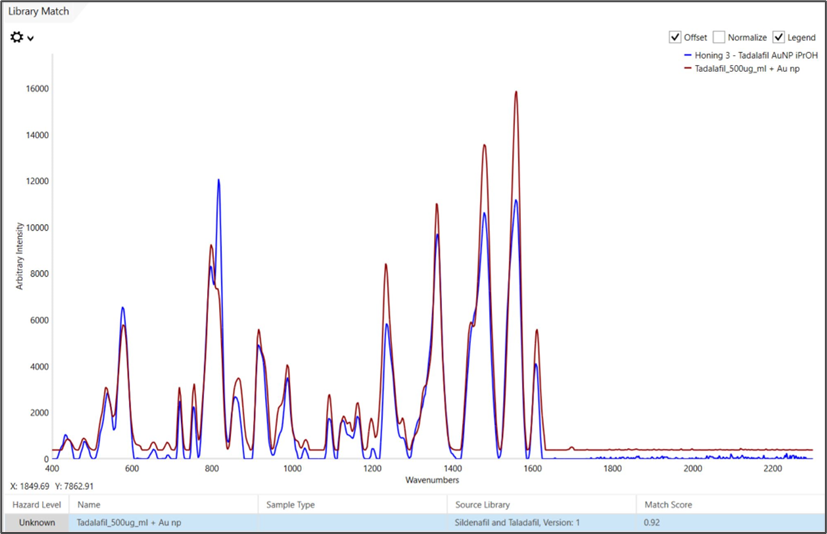
Figure 5. The spectrum of Honey 3 after a simple extraction and analysis with Gold NP colloid solution and MISA. This honey sample spectrum is overlaid with the reference tadalafil SERS spectrum with which it was matched. Image Credit: B&W Tek
Figure 5 shows sample 3 with the excellent confidence offered by a high HQI score through library matching routines in MISA Cal software. The signal of sample 4 is lower than that of sample 3, as shown in Figure 6. This aligns with the lower known concentration of tadalafil in the sample.
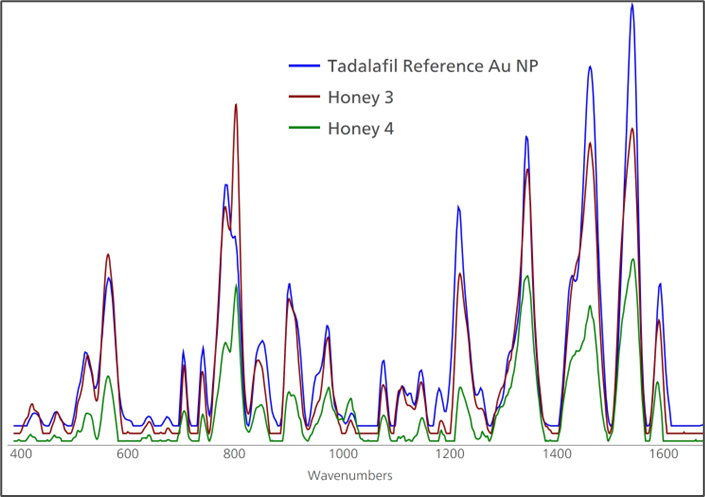
Figure 6. The presence of undeclared tadalifil in honey is confirmed through favorable comparison of honey samples with a tadalifil Gold NP SERS reference spectrum. Image Credit: B&W Tek
In all four honey samples, the presence of sildenafil and/or tadalafil is determined by SERS analysis. Sample preparation time averages 10 minutes per sample, with most of the time dedicated to dissolving the honey in the NaOH solution. MISA itself necessitates a maximum measurement time of 10 seconds.
Conclusion
SERS is gaining popularity as an analytical tool due to its low cost, high sensitivity, and the ability to offer a portable, quick technique for in-situ trace detection. Identification of illegal adulterants no longer necessitates high-resource laboratory techniques, such as mass spectrometry and liquid or gas chromatography. Valuable features of the Metrohm’s SERS solutions include:
- Lack of interference from the matrix
- High selectivity for target adulterants
- Low-cost analysis that mitigates the expense of consumables, laboratory equipment, and highly-trained personnel
- Simplicity of both sample pre-treatment and analysis
As both internal and external research demonstrate, SERS delivers target identification in the presence of expected excipients with minimal sample processing. This helps protect unsuspecting consumers from harmful adulterants.
- Rizzo, S.; Weesepoel, Y.; Erasmus, S.; Sinkeldam, J.; Piccinelli, A. L.; Ruth, S. van. A Multi-Analyte Screening Method for the Rapid Detection of Illicit Adulterants in Dietary Supplements Using a Portable SERS Analyzer. Heliyon 2023, 9 (8). https://doi.org/10.1016/j.heliyon.2023.e18509.
- Commissioner, O. of the. FDA Warns Four Companies for Selling Tainted Honey-based Products with Hidden Active Drug Ingredients. FDA. https://www.pharmaceuticalprocessingworld.com/fda-warns-four-companies-over-selling-honey-tainted-with-prescription-drugs/#:~:text=FDA%20has%20sent%20warning%20letters,the%20products%20through%20laboratory%20testing. (accessed 2024-01-08).

This information has been sourced, reviewed and adapted from materials provided by B&W Tek.
For more information on this source, please visit B&W Tek.