Near-infrared spectroscopy, known as NIR spectroscopy or NIRS, is a long-established analytical technique. For over 30 years, it has proven to be a fast and reliable method for measuring chemical and physical properties in solids and liquids.
This article introduces how NIR spectroscopy works alongside a presentation of the technique’s advantages and versatility.
How Does NIR Spectroscopy Work?
NIR spectroscopy analyzes how light and matter interact, generating a spectrum. Generally, light is described by wavelength rather than applied energy when spectroscopic methods are applied.
NIR spectroscopy functions in the wavelength range of 780 to 2500 nm, which is the near-infrared region of the electromagnetic spectrum. Simply put, a NIR spectrometer measures how much light is absorbed at various wavelengths in the NIR region.
It should be noted that near-infrared and mid-infrared are two different wavelength ranges.
NIRS is a secondary technique, meaning a prediction model is required before performing the technique. This is comparable with HPLC. To detect or quantify a substance using HPLC, standard solutions of the substance should be prepared and measured to create a calibration curve.
Similarly, with NIRS, a number of spectra with known concentrations or known parameter values that were acquired using a primary method, such as titration, must be measured. From this spectra, a prediction model is created using chemometric software (e.g., the Metrohm Vision software).
Once the prediction model has been generated, routine analysis of unknown samples can be initiated.
There are a number of functional groups, including -CH, -NH, -OH, and -SH to which NIR spectroscopy is particularly sensitive. Therefore, first quantifying the sample’s chemical parameters, such as water content (moisture), hydroxyl value, acid number, and amine content, to name a few, is an ideal approach.
The interaction between light and matter depends upon the matrix of the sample itself, facilitating the identification of physical and rheological parameters, including density, intrinsic viscosity, melt flow rate, and particle sizing.
Measuring Methods for Solid and Liquid Samples
To understand the advantages of NIR technology, understanding how NIR spectra is measured is key NIR spectroscopy facilitates the analysis of a wide variety of different samples. Accordingly, the sample type determines what instrument will need to be used.
There are a number of measurement methods available for the different sample types, ranging from clear liquids to opaque pastes and powders. Knowing which measurement method, sampling module, and what accessories to use is essential when developing effective NIR methods.
Below, the different methods for a variety of sample types (diffuse reflection, diffuse transmission, transflection, and transmission) are described.
Measuring Methods for Solid Samples
Diffuse Reflection: Cream, Granulates, Paste, Coarse and Fine Powders
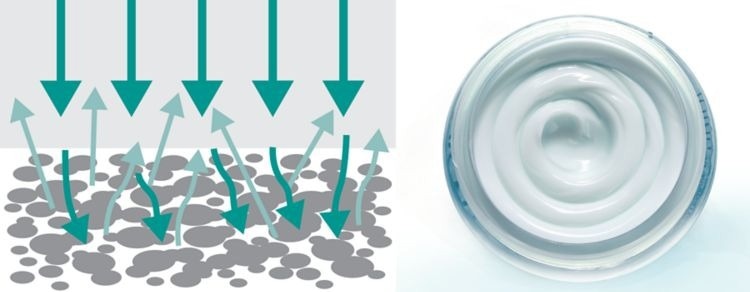
Image Credit: Metrohm Middle East FZC
In this interaction, NIR light permeates through the sample and any unabsorbed NIR energy is reflected back to the detector. This method is extremely useful when measuring solid samples without any sample preparation.
Diffuse Transmission: Tablets and Capsules

Image Credit: Metrohm Middle East FZC
Similar to diffuse reflection, the NIR light permeates and interacts with the sample. This light scatters throughout the sample due to how it interacts with the particles. Before reaching the detector, the unabsorbed NIR light is transmitted back through the sample. This method is well-suited to measuring solid dosage forms without having to prepare the sample.
Example for Measuring a Solid Sample
Solid samples (e.g., powders) must be secured within an appropriate container or vial and placed on the window as illustrated.
The NIR radiation is emitted in an upward direction partially reflected by the sample toward the detector, which is located underneath the sample vessel plane. The measurement run time is just 45 seconds, and, once complete, a result is displayed.
As the reflected light holds all the relevant sample data, this measurement technique is known as diffuse reflection.

Image Credit: Metrohm Middle East FZC
Measuring Methods for Liquid Samples
Transflection: Liquids and Gels
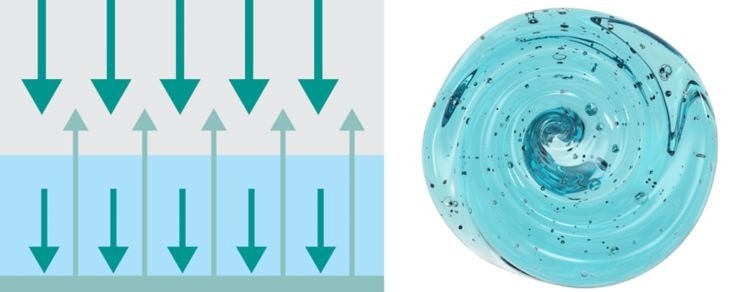
Image Credit: Metrohm Middle East FZC
This measurement method combines the techniques of transmission and reflection. A reflector is positioned at the rear of the sample. Any unabsorbed NIR light is reflected back to the detector via the reflector. This technique is ideal for measuring liquid samples.
Transmission: Liquids
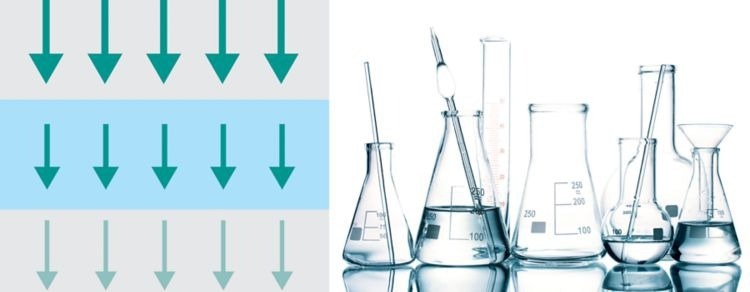
Image Credit: Metrohm Middle East FZC
Here, the sample is positioned between the detector and the NIR light source. NIR light passes through the sample, and the unabsorbed NIR energy is transmitted to the detector. This technique is most useful when measuring clear liquid solutions or suspensions.
Example for Measuring a Liquid Sample
As illustrated, when running NIR analysis of liquid samples, a vial or cuvette is inserted into the sample holder. The process run time is 45 seconds after which a result is generated.
Here, the NIR radiation passes through the solution before being transmitted to the detector. Hence, this measurement technique is called transmission.

Image Credit: Metrohm Middle East FZC
Advantages of NIR Spectroscopy
The NIR spectrum acquisition procedure demonstrates two clear advantages of NIR spectroscopy: speed and simplicity.
The main advantages of NIR analyses are listed below:
- Rapid measurement technique – results generated in under a minute.
- Sample preparation not required – solids and liquids can be used in true form.
- Low cost per sample – no chemicals or solvents required.
- Environmentally-friendly – no waste produced.
- Non-destructive – preserves precious samples for reused after analysis.
- Easy to operate – user friendly system
NIR Spectroscopy Applications
The versatility of NIR Spectroscopy means it can be used for a wide variety of applications. NIR analysis can be utilized for both the analysis of chemical and physical parameters across a number of applications in the chemical, food, animal feed, polyol, polymer, pharma, pulp and paper, paint, petrochemical, and petrofuel industries.
NIR instruments are typically used for quality assurance and quality control, identifying and validating raw materials or chemical compositions, process control, in situ reaction monitoring, and real-time screening.

Image Credit: Metrohm Middle East FZC
A variety of application examples are outlined in the below articles:
Polymers: Density of Polyethylene (PE); Melt Flow Rate; Intrinsic Viscosity
Chemical: Hydroxyl number of polyols
Petrochemical: Research Octane Number (RON) of gasoline; cetane index for diesel
Oils and Lubricants: Total Acid Number (TAN)
Pharma: Water content of lyophilized products; content uniformity in tablets
Personal care: Moisture content and active ingredients in creams
Near-infrared spectroscopy is a trusted method for measuring chemical and physical properties in solids and liquids. This rapid, analytical technique is easy to use, meaning staff without any laboratory education for routine analysis can successfully generate dependable results.
Learn more about how NIR spectroscopy can be readily implemented into your laboratory workflow: https://www.metrohm.com/en_ae/products/near-infrared-spectroscopy.html

This information has been sourced, reviewed and adapted from materials provided by Metrohm Middle East FZC.
For more information on this source, please visit Metrohm Middle East FZC.