 By Taha KhanReviewed by Lexie CornerMar 7 2024
By Taha KhanReviewed by Lexie CornerMar 7 2024
Gas sorption refers to the interaction between gas molecules and a solid surface by adsorption, where gas molecules adhere to the surface, and desorption, where they detach and return to the gas phase.
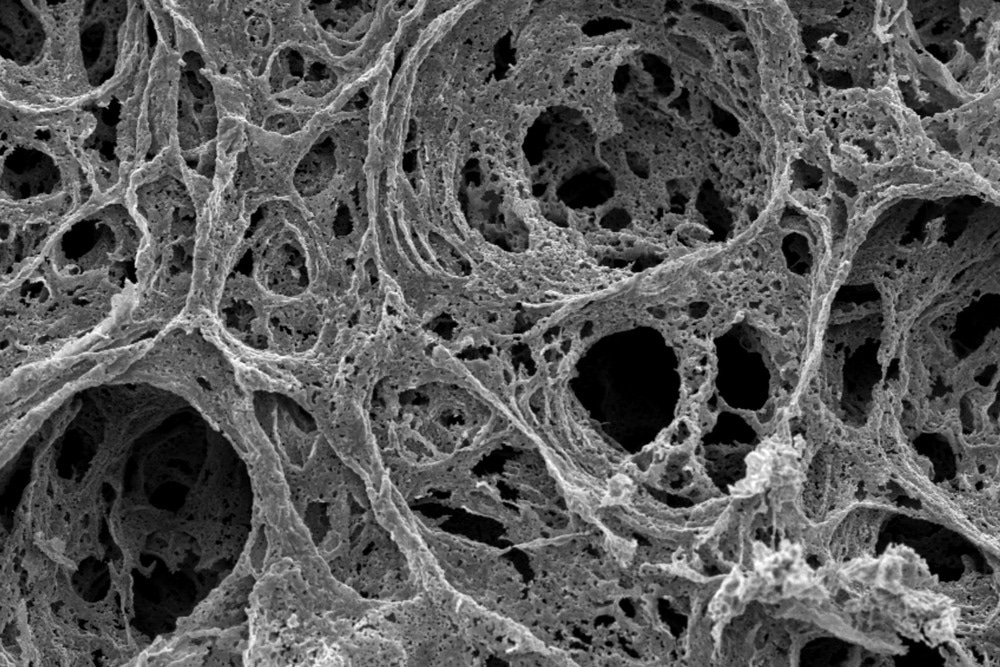
Image Credit: LuYago/Shutterstock.com
Factors Influencing Gas Sorption
Several factors, such as surface area, pore size distribution, temperature, pressure, and the nature of both the gas and porous material, influence the rate and extent of gas sorption.
The size and distribution of pores significantly impact the behavior of gas molecules and their sorption. The sorption rate of large surface areas is higher as they provide more space for interaction between gas molecules and pores.
Similarly, temperature and pressure directly influence the movement and kinetic energy of molecules. Higher temperatures typically decrease adsorption, while higher pressure generally increases it.
What is Gas Sorption Analysis?
Gas sorption analysis is a technique used to characterize the properties of solid materials by quantifying the interactions between gas and solid phases. It provides valuable information about the materials' surface area, pore size, shape, and chemically active sites.
Nitrogen, carbon dioxide, and argon are among the gases commonly used for sorption analysis, each serving specific purposes based on their interaction with the material under investigation.
How Do Sorption Rates Differ Among Materials?
Different materials exhibit varying degrees of porosity, significantly impacting their sorption rates. For instance, high porosity materials, such as activated carbons and metal-organic frameworks (MOFs), possess large surface areas and extensive pore volumes, enabling them to adsorb substantial quantities of gas molecules. In contrast, materials with lower porosity, such as certain zeolites or mesoporous silica, may exhibit reduced sorption capacities due to fewer available adsorption sites.
Researchers employ various techniques to analyze differences in sorption rates among materials with varying porosity. For instance, the Brunauer-Emmett-Teller (BET) method quantifies surface area, the Barrett-Joyner-Halenda (BJH) or Density Functional Theory (DFT) analysis characterizes the distribution of pore sizes within a material, and kinetic studies provide sorption rates over time.
In a recent study conducted in 2022, researchers explored the potential of Raman spectroscopy as a novel technique for characterizing gas sorption in translucent porous materials, crucial for applications like carbon capture and hydrogen processing. They demonstrated Raman spectroscopy's efficacy in determining adsorption capacity and selectivity by examining the adsorption performance of silica ionogels under pressure. This method offers rapid screening capabilities, requiring only minimal sample amounts.
The study validated spectroscopic results with traditional gravimetric measurements, showing good agreement. Unlike conventional methods, Raman spectroscopy can discern between different gas species within a mixture, enabling efficient assessment of an adsorbent's suitability for gas separation tasks. According to the study, further enhancements to experimental procedures could refine measurement uncertainties and expand the technique's applicability to a wider range of gas mixtures and temperatures.
Why is Gas Sorption Analysis Important?
Gas sorption analysis is an important tool and has various applications. In environmental science, gas sorption analysis contributes to the design of adsorbents for pollutant removal from air and water. Gas sorption analysis is also used in the development of adsorbent materials for hydrogen or methane storage. Similarly, in catalysis, understanding gas adsorption on porous catalyst materials is crucial for enhancing reaction rates and selectivity.
Recent Advancements in Gas Sorption Analysis
Understanding Mesoporous Glass with the Serially Connected Pore Model
In a 2019 study, researchers investigated nitrogen sorption and water phase transitions in mesoporous glass with irregular pore structures. They employed a newly developed model called the serially connected pore model (SCPM), which integrates structural disorder by considering coupling between nucleation and phase growth in disordered mesopores.
Contrary to prevailing independent pore models, SCPM accurately describes boundary and scanning transitions, showing sensitivity to microscopic fluid phase distribution.
The study demonstrated SCPM's ability to quantify structural information from gas sorption and cryoporometry data. Results suggest that SCPM is effective in understanding phase equilibria in geometrically disordered mesopores, offering a robust tool for structural analysis in porous materials.
Cooperative Sorption Isotherm for Porous Materials
In another 2021 study, researchers addressed the challenge of understanding gas sorption in porous materials by bridging statistical thermodynamics with experimental sorption isotherms. They introduced a cooperative sorption isotherm derived from rigorous statistical thermodynamics, offering a novel approach to analyzing microporous and mesoporous materials without relying on assumptions about adsorption sites or pore geometry.
Unlike traditional models, their approach directly connects molecular interactions with sorption behavior, allowing for a deeper understanding of sorption mechanisms. The researchers demonstrated its effectiveness in capturing cooperative sorption phenomena by fitting their model to experimental data on water vapor adsorption on activated carbon fibers. This study provides a clear framework for interpreting sorption isotherms, facilitating a more precise characterization of porous materials.
Conclusion
Gas sorption analysis is vital for understanding material properties crucial in the environmental, energy, and catalysis fields. Recent studies, including SCPM for mesoporous glass and a cooperative sorption isotherm, offer innovative approaches to accurately characterize porous materials' structure and behavior, advancing sorption analysis techniques for diverse applications.
More from AZoM: How is Porous Titanium Used?
References and Further Reading
Enninful, HR., Schneider, D., Hoppe, A., König, S., Fröba, M., Enke, D., Valiullin, R. (2019). Comparative gas sorption and cryoporometry study of mesoporous glass structure: Application of the serially connected pore model. Frontiers in Chemistry. doi.org/10.3389/fchem.2019.00230
Ilkaeva, M., Vieira, R., Pereira, JM., Sardo, M., Marin-Montesinos, I., Mafra, L. (2023). Assessing CO2 Capture in Porous Sorbents via Solid-State NMR-Assisted Adsorption Techniques. Journal of the American Chemical Society. doi.org/10.1021/jacs.3c00281
Lipinski, G., Jeong, K., Moritz, K., Petermann, M., May, E. F., Stanwix, P. L., Richter, M. (2022). Application of Raman Spectroscopy for Sorption Analysis of Functionalized Porous Materials. Advanced Science. doi.org/10.1002/advs.202105477
McLaren, R. L., Laycock, C. J., Brousseau, E., Owen, GR. (2021). Examining slit pore widths within plasma-exfoliated graphitic material utilizing Barrett–Joyner–Halenda analysis. New Journal of Chemistry. doi.org/10.1039/D1NJ01702K
Shimizu, S., Matubayasi, N. (2021). Cooperative sorption on porous materials. Langmuir. doi.org/10.1021/acs.langmuir.1c01236
Sinha, P., Datar, A., Jeong, C., Deng, X., Chung, YG., Lin, LC. (2019). Surface area determination of porous materials using the Brunauer–Emmett–Teller (BET) method: limitations and improvements. The Journal of Physical Chemistry C. doi.org/10.1021/acs.jpcc.9b02116
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.