This article is based on a poster originally authored by Rui Santos, Oliver Buettel, and Siqi Sun.
The goal was to complete a geological profile on five ore specimens utilizing the quickest method to analyze 44 elements with inductively coupled plasma mass spectrometry (Q ICP MS) in three minutes. The suitability of a one-step microwave-assisted dissolution method was examined.
A mixture of HNO3/HCl/HF (8:1:1 v/v/v) combined with an MW digestion procedure (T max= 230 ºC, Pmax= 1400 W; t(ramp)= 20 min) provided a complete chemical profile of the specimens.
The scientific results for certified reference material (NCS DC 73325, a Soil) were utilized for quality control. Many of the elements measured closely matched the certified values with recoveries in the 90-110% range and RSD <5%. The RSD values were above 10% for Mg, Al, Se, Sr, and Cd elements measured in low solution concentrations.

 Download the Poster
Download the Poster
Instrumentation
The scientific analyses were completed utilizing the PlasmaQuant MS, which includes the integrated collision/reaction cell (iCRC) technology for removing polyatomic species created in the plasma while improving accuracy and precision.
The ICP-MS system was installed to a CETAC ASX560 autosampler, an ASXPress Plus CETAC injection valve, a Scott-type spray chamber with a Peltier chiller, and a MicroMistTM nebulizer; 0.1 mL/min was utilized to quantify 44 elements in five ore specimens and one Certified Reference Material soil (NCS DC 73325) for quality control.
The installed equipment rapidly quantified 44 elements in less than three minutes. The soil specimens were dissolved using a closed container microwave-assisted (speed wave XPERT) system.
Table 1 summarizes the instrument operating parameters, including iCRC modes in which the problem of spectroscopic interference was removed by utilizing helium and hydrogen gases on the first-row transition metals.
Table 1. PlasmaQuant MS Elite operating conditions. Source: Analytik Jena US
| Parameter |
Specification |
| Plasma Gas Flow |
10.5 L/min |
| Auxiliary Gas Flow |
1.50 L/min |
| Sheath Gas Flow |
0.00 L/min |
| Nebulizer Gas Flow |
1.06 L/min |
| Sampling depth |
5.0 mm |
| Plasma RF Power |
1.40 kW |
| Ramp Rate |
20 rpm – black/black PVC pump tubing (<1 mL/min) |
| Stabilization delay |
10 s |
| iCRC Gas Setting |
He 120 mL/min: 31P, 44Ca, 45Sc, 52Cr, 56Fe, 71Ga, 89Y, 95Mo, 105Pd, 112Cd, 115In, 121Sb, 128Te, 139La, 140Ce, 195Pt, 197Au and 200Hg |
| No Gas: 7Li, 9Be, 11B, 23Na, 24Mg, 27Al, 39K, 49Ti, 55Mn, 59Co, 60Ni, 65Cu, 66Zn, 86Sr, 90Zr, 109Ag, 118Sn, 137Ba, 182W, 205Tl, 206+207+208Pb, 209Bi and 238U |
| H2 120 mL/min: 51V, 75As and 78Se |
| Dwell Time |
10 ms (No Gas) and 20 ms (iCRC) |
| Scan per Replicate |
25 (peak hopping, 1 pt/peak) |
| No. of Replicates |
6 |
| Sample uptake time |
0 s – OneFAST Sample Introduction system used |
| Internal Standards |
159Tb and 175Lu at 5 μg/L, interpolate correction |
Sample Preparation and Reagents
The Certified Reference Material soil (NCS DC 73325) and the ore specimens were placed in the clean speed wave XPERT dissolution system container and mixed with the reagent (s), as indicated in Table 2.
Table 2. Digestion method parameters used by speedwave XPERT microwave digestion system. Source: Analytik Jena US
| Parameter |
Specification |
| MW procedure |
HNO3/HCl/HF (8:1:1 v/v/v) |
| HNO3 |
8 mL |
| HCl |
1 mL |
| HF |
1 mL |
| Sample amount |
0.1 g dried and sieved |
| Vessel |
PM60 |
| Heating Stage 1 / Time |
10 ºC /min |
| Heating Stage 2 / Time |
175 ºC / 10 min
230 ºC / 20 min |
| Cooling / Time |
25 ºC / 30 min |
| Final Volume |
50 mL with ultrapure H2O filtered through |
| ICP-MS prior dilution |
1:10 with 1% v/v HNO3 |
Results and Discussion
Figure 1 shows good 90-110% recoveries for many of the quantified elements utilizing the system developed. The exceptions were Mg, Al, Ca, Sr, and Ba. Al was found in lower concentrations in solutions containing HF because of the aluminum fluoride precipitation.
Examining two major elements (Na and K) indicated low recoveries when HF was used. This could be because the HF was limited to 1 mL to dissolve the SiO2 present in more than 32% of the NCS DC 73325 reference material. This could result in part of the Mn and Fe still being attached to the remainder of the SiO2.
The ore specimens had a lower amount of SiO2 than expected, less than the reference material. The procedure implemented produced precise and accurate data. The RSDs were approximately 5% or less for most of the elements.
It is important to prevent the formation of insoluble fluorides in the final specimen solution caused by the higher pressure attained in a MW container and to avoid the safety issues related to handling an extremely corrosive and toxic acid (HF).
As a result, a new method developed with the NH4HF2 −open-vessel acid digestion will be examined as the high boiling point of NH4HF2 (239.5 °C) provides an elevated dissolution temperature that efficiently dissolves refractory minerals.
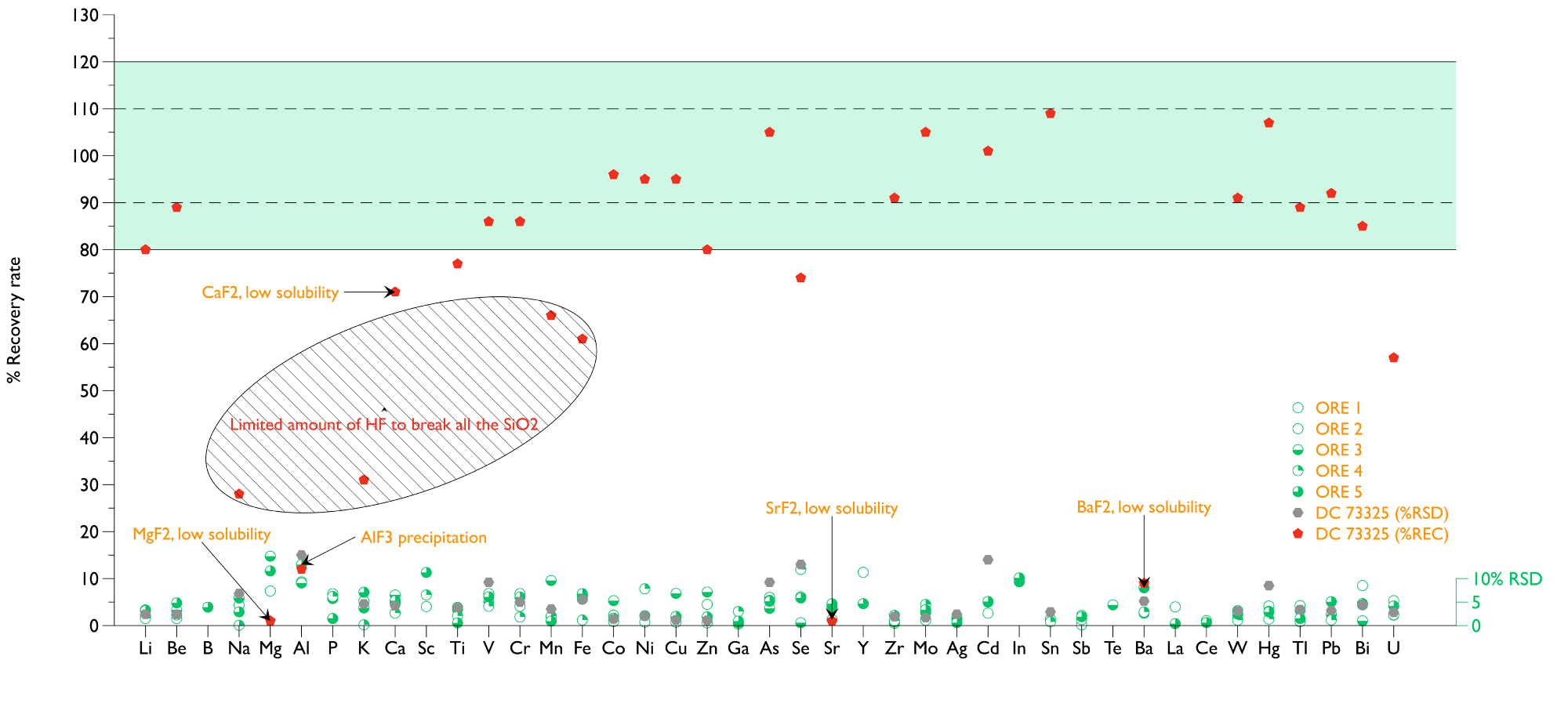
Figure 1. Recovery rate and precision NCS DC 73325 and 5 Ore samples. Pd, Pt, and Au were below MDL. Image Credit: Analytik Jena US
Conclusions
An ICP-MS method providing quick and durable results was developed to analyze 44 elements of interest in ore specimens. A speed wave microwave digestion system dissolved the samples in <3 minutes, utilizing only half the argon gas volume compared to other ICP-MS equipment.
Some of the elements formed insoluble fluoride precipitates in the MW containers. A new open container method utilizing NH4HF2 will be performed to dissolve refractory and silicate minerals (e.g., albite, biotite, diopside, forsterite, hedenbergite, hornblende, kaolinite, plagioclase).

 Download the Poster
Download the Poster

This information has been sourced, reviewed and adapted from materials provided by Analytik Jena US.
For more information on this source, please visit Analytik Jena US.