Sponsored by PerkinElmerReviewed by Olivia FrostJun 20 2024
In a competitive industry, PerkinElmer increases productivity in pharmaceutical laboratories and improves sample analysis throughput by completing two analytical techniques at the same time. The Pyris™ STA 9 Simultaneous Thermal Analyzer (STA) can simultaneously perform Thermogravimetric Analysis and Differential Scanning Calorimetry.

Image Credit: Shutterstock/Gorodenkoff
In the "Early Drug Discovery Phase" of pharmaceutical development, quick thermal analysis using a small sample size is essential when only a minimal amount of synthesized drug candidate is available. The sample amount could be less than 3 mg.
Due to the urgency of identifying potential drug candidates, analytical results must be provided on the same day. The Pyris STA 9, with its sensitivity of 0.1 μg, allows for the use of minimal sample material while obtaining reproducible results in half the time.
This article examines three typical drug-discovery-type pharmaceutical materials using Simultaneous Thermal Analysis, specifically TGA-DSC.
- Sample A: Free-base, small-molecule crystalline powder
- Sample B: HCl salt of Sample A, monohydrate
- Sample C: Mesylate salt of Sample A, trihydrate
Experimental
The analysis was conducted on a PerkinElmer Pyris STA 9, using alumina ceramic pans (N5200040) and a standard furnace.
The furnace of the instrument was calibrated using the melting temperature of a metal reference material, specifically a single-point indium melting event, to calibrate both temperature and heat flow.
The instrument's balance was calibrated using a certified weight. Dry nitrogen, with a flow rate of 30 mL/minute, was used as the sample purge gas. The large Sample A was tested to demonstrate the Pyris STA 9's ability to handle various sample sizes. The temperature program ranged from 30 ℃ to 350 ℃, with a scanning rate of 10 ℃ per minute.
Sample Weights:
- Sample A: 11.679 mg
- Sample B: 4.829 mg
- Sample C: 5.562 mg
Results
Sample A (Figure 1) is characterized by the Pyris STA 9 and is shown as the Red DSC thermal curve and the Blue TGA weight loss curve. Sample A is a free base, small-molecule crystalline powder (11.679 mg). The DSC curve shows that a crystalline melt is apparent and defined by a peak temperature of 228.37 °C.
Following the melt transition, the baseline returns to a marginally lower position than the pre-melt baseline. The post-melt baseline alters the slope as the sample starts decomposition. The DSC exothermic decomposition peak at 287.2 °C aligns with the TGA extrapolated onset temperature of 287.2 °C over decomposition.
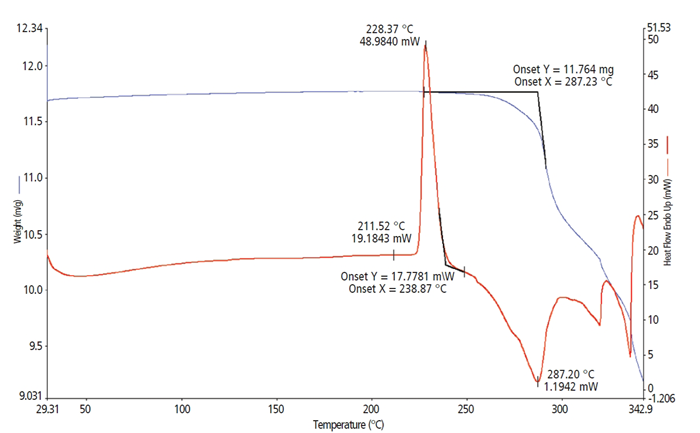
Figure 1. Sample A. Image Credit: PerkinElmer
Sample B (Figure 2) (4.89 mg), an HCl salt of Sample A monohydrate, shows a weight loss of water after heating of 0.168 mg. The water lost is measured using a basic delta Y calculation on the TGA curve. It is a close match to the calculated value from the stoichiometric 1:1 ratio for a monohydrate. The coordinating DSC thermal curve shows a matching endothermic reaction that starts directly following the start-up transient and ends at 111.81 °C.
The sample continues heating until an endothermic event starts right at the beginning of decomposition. This is observed by the start of the endothermic DSC event and its relationship to the beginning of the weight loss curve. The sample then experiences a sudden decomposition exothermic event, which starts at 291.64 °C as shown by the DSC Peak temperature.
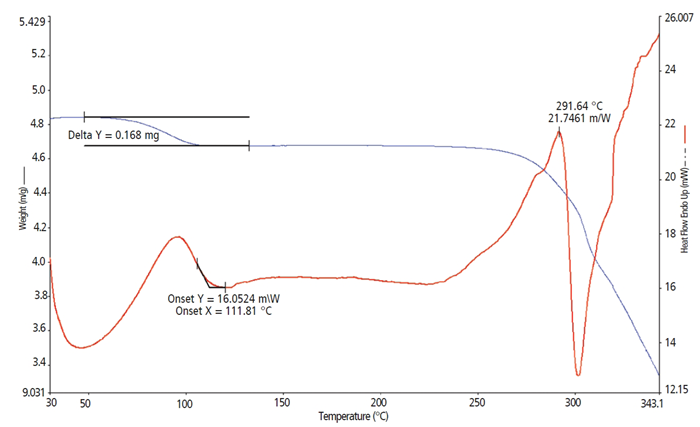
Figure 2. Sample B. Image Credit: PerkinElmer
Sample C (Figure 3) is (5.562 mg) Mesylate salt of Sample A and exists as a trihydrate. The TGA curve exhibits surface water weight loss of 0.410 mg and decomposes above 250 °C. As anticipated, the DSC thermal curve shows three peaks.
The first DSC endothermic peak is caused by the release of hydration water from the crystalline lattice. This corresponds to the TGA curve's surface water weight loss of 0.410 mg. The series of exothermic and endothermic events that occur between 215 °C and 270 °C represent a crystal-crystal transformation with heating.
The first endothermic peak represents a crystalline melt (an anhydrous crystalline phase as the hydration water was removed prior); the next peak at 236.73 °C is an exothermic event and is a recrystallization of the melted material to form a new crystalline structure, which is immediately followed by the melting of the same crystalline form.
This melt-recrystallization-melt event series is also known as a polymorph conversion (phase transition) process. It is a common characteristic of small-molecule organic crystals. All of the above happens just prior to the last decomposition.
Conclusion
The Pyris STA 9 offers the productivity that pharmaceutical companies and other industries seek by combining DSC and TGA thermal techniques, providing reproducible results in half the time.
Designed for both routine and research applications, the Pyris STA 9 Simultaneous Thermal Analyzer utilizes advanced sensor technology to deliver higher accuracy and quality results. The SaTurnA™ sensor and the proven compact furnace ensure better temperature control, more consistent measurements, and the fastest cool-down time.
To further enhance productivity, the Pyris STA 9 features an easy-to-load vertical system that can be equipped with an autosampler for higher throughput and unattended sample analysis.
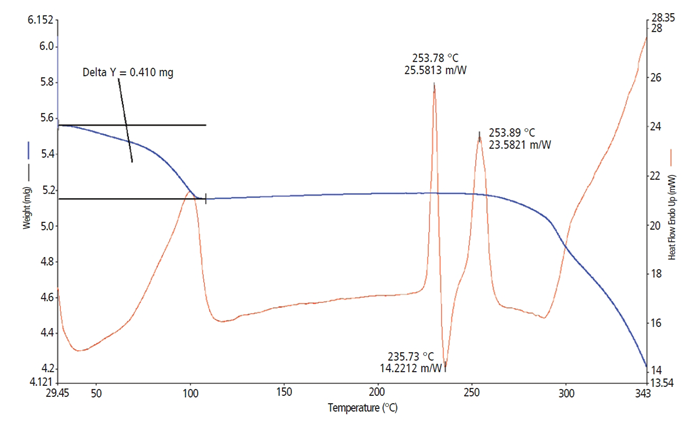
Figure 3. Sample C. Image Credit: PerkinElmer

This information has been sourced, reviewed and adapted from materials provided by PerkinElmer.
For more information on this source, please visit PerkinElmer.