Hydrogen sulfide is typically found in natural gas and, even at very low concentrations, can be fatal.2,3 Usually, this compound is sequestered via H2S scavengers, such as triazines.4 A common reaction pathway is displayed in Figure 1, wherein triazine (1) reacts sequentially with two equivalents of H2S to essentially form 3 via intermediate 2.5 In suitable conditions, the two equivalents of monoethanolamine (MEA, 4) produced during these transformations can react further with H2S.6
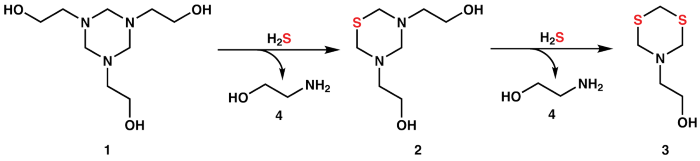
Figure 1. Proposed triazine (1) reaction pathway with H2S to sequentially form thiadiazine (2) and dithiazine (3), along with two equivalents of MEA (4). Image Credit: Nanalysis Corp.
The formation of dithiazine (3) solid deposits, which result from the scavenger (1) being overspent, can lead to several issues. This results in the need to characterize the reaction products to establish the remaining scavenging capacity of the triazine (1).7
While NMR easily characterizes the products generated by such reactions8, the high upfront and recurring costs of conventional high-field instruments (1H operating frequencies ≥300 MHz), in combination with their size and requirements for skilled staff to operate and maintain, can make it challenging to incorporate these instruments into many industrial applications.
The rising popularity and improved performance of high-resolution benchtop NMR spectrometers have made it easier for laboratories to access this technique, which benefits various industries. Benchtop NMR spectrometers are, therefore, good for monitoring these types of transformations.
In the study presented in this article, one unspent scavenger and four spent scavengers (acquired from the field) were assessed using 1H and 13C NMR spectroscopy. Controlled exposure to H2S also enabled the characterization of its reactivity with the scavenger over time.
Evaluating the reactivity of the triazine (1) with varying amounts of H2S was conducted by feeding a mixture of gas through a pH-controlled solution derived from the scavenger and subsequently measuring the “break-through” time. At this point, it was determined that the scavenger was 100 % spent.
From here, the tests were repeated to prepare scavenger solutions, which were 80 % and 50 % spent. The results revealed that the spent scavenger samples appeared almost identical to those taken from the field, including the residual presence of 1. This implies that it is not entirely consumed as previously thought. Interestingly, there was minimal presence of 3 in the 50 % spent solution, with only small amounts starting to appear in the 80 % spent solution. Indeed, 3 only became a significant reaction product once breakthrough had been achieved. These studies reveal that benchtop NMR instruments can be effectively employed to monitor the reactivity of 1 toward H2S. They also demonstrate that amounts of both 1 and 2 are still present at the break-through point, suggesting that another component inhibits further reactivity.
This article was adapted from work published by Brown in Magnetic Resonance in Chemistry.1 The reader is advised to read the complete publication for additional information on the work discussed, including figures of NMR spectra and a detailed discussion.
References and Further Reading
- Brown, B. A. Magn. Reson. Chem. 2020, 58, 1249–1255.
- Sour Gas:https://www.aer.ca/understanding-resource-development/resource-development-topics/sour-gas#:~:text=Sour%20gas%20is%20natural%20gas,be%20used%20to%20recover%20sulphur. (accessed April 10, 2024).
- Guidotti, T. L. Int. Arch. Occup. Environ. Health 1994, 66, 153–160.
- Salma, T.; Briggs, M. L.; Herrmann, D. T.; Yelverton, E. K. Hydrogen Sulfide Removal from Sour Condensate Using Non-Regenerable Liquid Sulfide Scavengers: A Case Study. SPE Rocky Mountain Petroleum Technology Conference. Society of Petroleum Engineers: Keystone, Colorado 2001, p. 5. https://doi.org/10.2118/71078-MS.
- Bakke, J. M.; Buhaug, J.; Riha, J. Ind. Eng. Chem. Res. 2001, 40, 6051–6054.
- Taylor, G. N.; Prince, P.; Matherly, R.; Ponnapati, R.; Tompkins, R.; Vaithilingam, P. Ind. Eng. Chem. Res. 2012, 51, 11613–11617.
- Taylor, G. N.; Matherly, R. Ind. Eng. Chem. Res. 2011, 50, 735–740.
- Buhaug, J. Investigation of the Chemistry of Liquid H2S Scavengers , Norwegian University of Science and Technology, Trondheim, Norway 2002

This information has been sourced, reviewed and adapted from materials provided by Nanalysis Corp.
For more information on this source, please visit Nanalysis Corp.