Method detection limits (MDLs) have been determined for the newly regulated HON (Hazardous Organic NESHAP (National Emission Standards for Hazardous Air Pollutants)) compounds, which validate selected ion flow tube mass spectrometry (SIFT-MS) as an effective solution for measuring these toxic volatile organic compounds (VOCs) and other environmental pollutants in ambient air, whether at the fence line or in a mobile setting.
SIFT-MS provides exceptional speed, sensitivity, and ease of use in environmental monitoring applications.

Image Credit: petrmalinak/Shutterstock.com
Introduction
Over 200 chemical factories in the United States manufacture chemicals that offer a considerable cancer risk to local communities. These are referred to as HON facilities.
In January 2024, the United States Environmental Protection Agency (EPA) issued a rule requiring fenceline monitoring of six harmful VOCs produced by these facilities: ethylene oxide (EtO), chloroprene, benzene, 1,3-butadiene, ethylene dichloride, and vinyl chloride.
The new laws specify action levels for these compounds as thresholds for annual average air concentrations; if these levels are exceeded at the fenceline, facilities must identify and resolve the source of pollution to effectively control hazardous emissions.
SIFT-MS enables real-time, direct monitoring of VOCs and inorganic compounds in the air. SIFT-MS has been utilized for environmental monitoring by governments and industries worldwide.
For example, South Korean government agencies have used SIFT-MS for years to monitor harmful VOCs at the federal and state levels.1
Recently, the US EPA adopted SIFT-MS for use in mobile laboratories and real-time mobile monitoring systems.
Syft Technologies developed comprehensive sampling methodologies for harmful VOCs in response to the new HON Rule. These approaches have yielded MDLs in the low- to mid-part per trillion by volume (pptV) range, demonstrating SIFT-MS as an excellent solution for monitoring these and other chemicals in ambient air. With SIFT-MS, users get real-time monitoring that will connect emissions to specific events, giving a deeper understanding of plant operations while still meeting regulatory requirements.
Method
SIFT-MS achieves real-time specificity by combining rapidly switchable reagent ions with diverse reaction processes that identify several chemicals in a single study.
Mass spectrometry detection, paired with library records of ion-molecule reaction rate constants, quantifies target substances accurately.
This study employs a SIFT-MS apparatus (Syft Tracer) powered by nitrogen carrier gas and equipped with a high-performance inlet (HPI).
The HPI allows direct sample analysis, which minimizes volatile loss. Samples were prepared by diluting reference standards in clean, humid air to achieve a constant flow at the concentration of interest (40-50% relative humidity). The samples were transported to the HPI using a flow past setup.
Library entries were utilized to generate one-minute techniques for each compound. The methods included all quick reactions for reactive compounds with multiple reagent ions.
The measurement time for procedures involving positive reagent ions (H3O⁺, NO⁺, and O2⁺) and negative reagent ions (O-, OH-, O2-, NO2-, and NO3-) was 2 minutes, divided into 1 minute for each phase.
MDLs were determined using the US EPA technique EPA 821-R-16-006.2 The presence of the isomer acetaldehyde affects the quantification of EtO in H3O⁺.3
The procedure tested here includes subtracting acetaldehyde due to its reactivity with NO⁺. The EtO MDL was tested with 2.5 ppbV acetaldehyde to ensure that the results indicated a realistic environmental sample.
Table 1. Action levels for HON compounds. Source: Syft Technologies
| Analyte |
Molecular Formula |
HON Action Level |
| (μg/m3) |
(pptV) |
| Benzene |
C6H6 |
9 |
2770 |
| 1,3-Butadiene |
C4H6 |
3 |
1335 |
| Choloroprene |
C4H5Cl |
0.8 |
217 |
| Ethylene oxide |
C2H4O |
0.2 |
109 |
| Vinyl chloride |
C2H3Cl |
3 |
1155 |
| Ethylene dichloride |
C2H4Cl2 |
4 |
990 |
*μg/m³ to pptV conversion at 20 °C.
Results
MDLs were calculated for additional environmentally significant substances in addition to HON chemicals. Tables 2 and 3 show the MDLs for each molecule that may be achieved in one minute using the SIFT-MS.
Table 2. Comparison of MDLs using SIFT-MS with HON compound action levels. Results are for one-minute methods using a single reagent ion, except for EtO. Source: Syft Technologies
| Compound |
MDL (pptV) |
HON Action Level (pptV) |
| Vinyl chloride |
460 |
1170 |
| 1,3-Butadiene |
60 |
1360 |
| Benzene |
50 |
2820 |
| Ethylene oxide |
105† |
110 |
| Ethylene dichloride |
260 |
990 |
†EtO MDL over 30 minutes in the presence of 2.5 ppbV acetaldehyde (see Discussion).
Table 3. MDLs for environmentally important compounds using SIFT-MS. Results are for one-minute methods using a single reagent ion. Source: Syft Technologies
| Compound |
MDL (pptV) |
| Toluene |
25 |
| Xylenes and ethylbenzene |
55 |
| Hydrogen sulfide |
125 |
| Trichloroethylene |
165 |
| Nitrogen dioxide |
65 |
| Sulfur dioxide |
200 |
| Trans-1,2-dichloroethylene |
100 |
| 1,3,5-trimethylbenzene |
65 |
| Ammonia |
595 |
| Acetaldehyde |
35 |
| Formaldehyde |
595 |
| Methyl bromide |
90 |
| n-butane |
250 |
| Acrolein |
120 |
Discussion
The MDLs in Tables 2 and 3 show the capabilities of single-compound SIFT-MS techniques performed over one minute (30 minutes for EtO). All HON chemicals have MDLs lower than the EPA's action limit for fenceline monitoring.
The new EPA requirements for HON compound fenceline monitoring require alternate monitoring methodologies to obtain MDLs at least one-third of the action level for the monitored compounds.
Extending the monitoring period easily achieves lower MDLs for any SIFT-MS approach. The improvement in MDL is roughly proportional to the inverse square root of the method length.
EtO is a fantastic illustration of how changing the method duration can drastically improve an MDL.
A large dataset of genuine EtO one-minute scan data was aggregated over time, allowing MDLs to be calculated for the aggregated datasets. For EtO, the MDL using a one-minute technique exceeds the HON action threshold. Figure 1 shows the improvement in EtO MDL achieved by increasing the procedure duration.
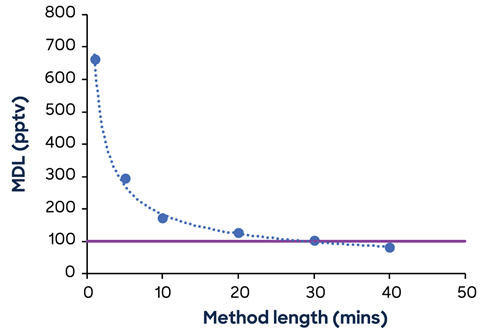
Figure 1. EtO MDL in the presence of 2.5 ppbV acetaldehyde as a function of method length. The HON action level is marked by the horizontal line. Image Credit: Syft Technologies
The MDL for EtO falls below the HON action threshold when the technique is longer than 30 minutes. To reach an MDL of one-third of the action level, the procedure length would need to be increased to around three hours.
This time frame is substantially shorter than the 24-hour average concentration determined by the traditional canister method for EtO, allowing many high-precision samples to be taken during the requisite 24-hour timeframe.
SIFT-MS has significantly better temporal resolution for all HON chemicals than the EPA-specified methods - passive and canister sampling - allowing for better source identification in case of a concentration above the action threshold.
According to the EPA protocol, MDLs can be determined by studying the calibration region with a considerable change in sensitivity, i.e., a break in the calibration's slope.
To corroborate the MDLs in Tables 2 and 3, measured at a single concentration, low-concentration calibrations were carried out for each chemical down to low pptV values. Figure 2 illustrates an example of 1,3-butadiene.
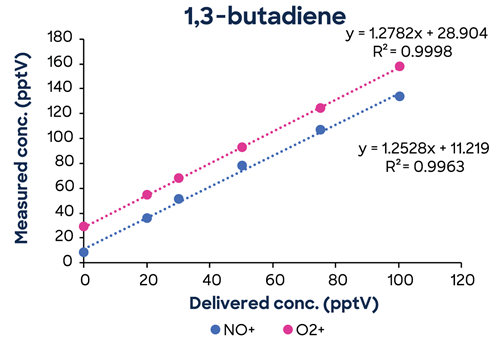
Figure 2. 1,3-Butadiene low concentration calibration showing excellent linearity down to low pptV concentrations. Image Credit: Syft Technologies
Concentrations of O2⁺ and NO⁺ show remarkable linearity down to less than 20 pptV. This high association demonstrates that the 1,3-butadiene MDL shown in Table 2 is reasonable and attainable.
Figure 3 shows additional low-concentration calibrations demonstrating SIFT-MS linearity down to low pptV values.
The measurement of Chloroprene's MDL was recently completed and recorded as 145 pptv which is below the HON action limit of 220 pptV. There is a lower action limit (83 pptV) for facilities collocated with neoprene productions sources. Similar to the EtO approach, the chloroprene MDL can be reduced to below this action level by extending method length to five minutes.
Syft Tracer's flexibility and ability to rapidly monitor multiple chemical species at low pptV concentrations make it an excellent choice for monitoring trace quantities of dangerous air contaminants. SIFT-MS is easy to automate, provides a simple data stream, and can operate unmonitored. It is the optimal solution for a facility aiming to go beyond basic compliance and achieve a higher standard of operational excellence.
SIFT-MS is well-positioned to address future regulatory issues thanks to its extensive database of ion-molecule reactions, including environmentally relevant chemicals.
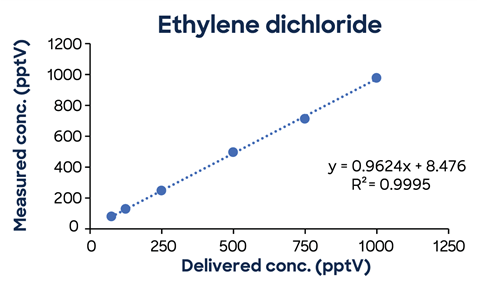
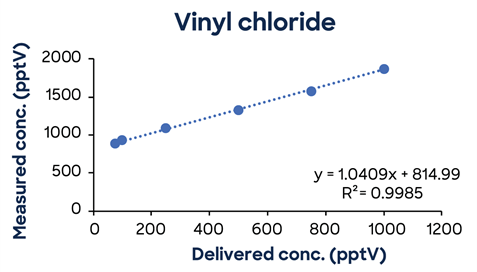
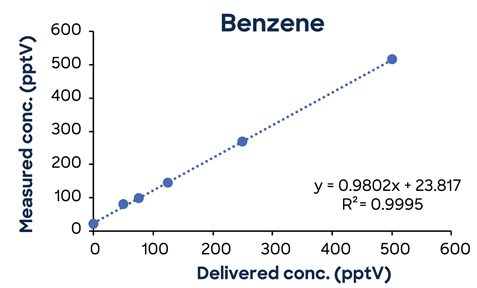
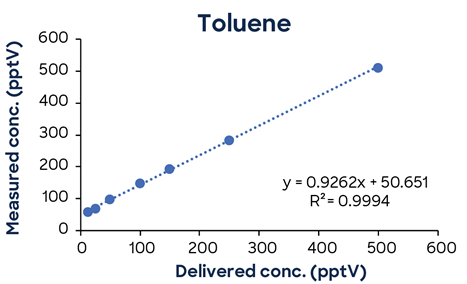
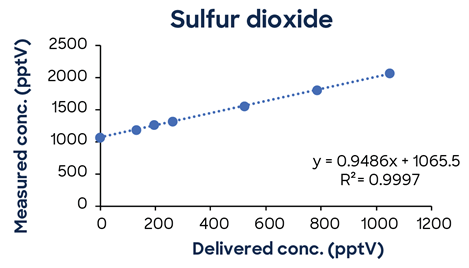
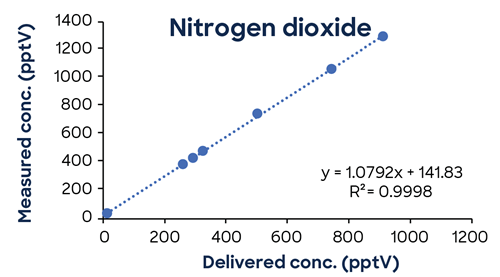
Figure 3. Low-concentration calibrations using simple one-minute methods exhibit excellent linearity down to low ppt concentrations. Image Credit: Syft Technologies
Conclusions
SIFT-MS measurements provide substantial advantages over traditional methods, which include lengthy sample collection times and off-site laboratory analysis.
Unlike traditional approaches, which can overlook short-term variations in pollutant concentrations, SIFT-MS gives rapid and continuous data, enabling the early detection of dangerous substances.
This feature improves the accuracy of source identification and allows for faster reactions to potential environmental concerns, resulting in more effective monitoring and public health protection.
How SIFT-MS Works
SIFT-MS employs soft chemical ionization (CI) to produce mass-selected reagent ions capable of rapidly reacting with and quantifying VOCs down to part-per-trillion concentrations (pptV).
Commercial SIFT-MS equipment now uses up to eight reagent ions (H3O⁺, NO⁺, O2⁺, O-, OH-, O2-, NO2-, and NO3-) derived from a microwave discharge in the air.
These reagent ions react with volatile organic compounds (VOCs) and other trace analytes in well-controlled ion-molecule reactions but not with air's primary components (N2, O2, and Ar).
This allows for the direct, real-time examination of air samples at trace and ultra-trace levels, with no pre-concentration required. Rapid switching between reagent ions gives great selectivity since the numerous reaction processes allow for independent measurements of each analyte.
Multiple reagent ions commonly decrease ambiguity from isobaric overlaps in mixtures with multiple analytes. The library entry's reaction rate constant (k) is employed to compute the compound concentrations.4
References and Further Reading
- Langford VS, Cha M, Milligan DB, Lee J (2023a). Adoption of SIFT-MS for VOC pollution monitoring in South Korea. Environments 10, 201. https://doi.org/10.3390/environments10120201
- EPA (2016). EPA 821-R-16-006 - Definition and Procedure for the Determination of the Method Detection Limit, Revision 2.
- Swift SJ, Dryahina K, Lehnert A-S, Demarais N, Langford VS, Perkins MJ, Silva LP, Omezzine Gnioua M and Španěl P (2023). Accurate selected ion flow tube mass spectrometry quantification of ethylene oxide contamination in the presence of acetaldehyde. Anal. Methods 15, 6435-6443. https://doi.org/10.1039/D3AY01036H
- Langford VS, Dryahina K, Španěl, P (2023b). Robust automated SIFT-MS quantitation of volatile compounds in air using a multicomponent gas standard. J. Am. Soc. Mass Spectrom. 34, 2630−2645. https://doi.org/10.1021/jasms.3c00312

This information has been sourced, reviewed and adapted from materials provided by Syft Technologies.
For more information on this source, please visit Syft Technologies.