Sponsored by Ossila LtdReviewed by Olivia FrostOct 11 2024
In sensitive research applications, ensuring very low levels of oxygen and moisture is paramount to the success of experiments. A glove box is often the chosen tool for this application, as it can maintain highly inert atmospheres. Yet even high-specification glove box systems, regardless of their design, allow some degree of moisture and oxygen ingress.
The choice of material used in constructing the glove box is critical in determining its effectiveness. Understanding the physical properties of commonly used materials can ensure you make the right choice when purchasing or building a glove box.
Understanding Ingress in Glove Boxes
Ingress, or permeation, is the diffusion of gases or liquids like oxygen and water vapor through a material. This unwanted diffusion can compromise the controlled environment inside a glove box, affecting the quality and integrity of sensitive experiments. The extent of this ingress is largely determined by the materials used to construct the glove box; materials with lower permeability rates are more effective in preventing contamination.
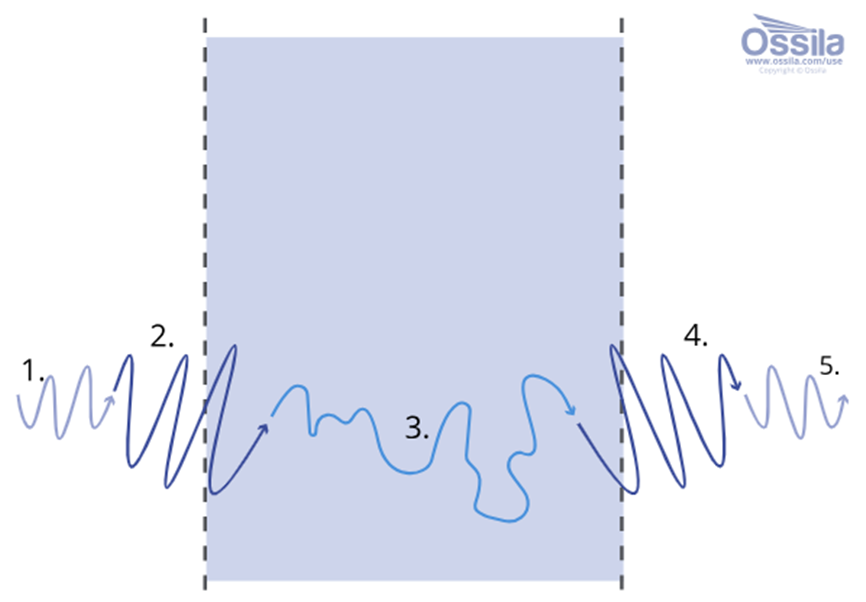
Image Credit: Ossila Ltd
The ingression process can be split into several different steps. The figure above shows the stages of a permeant traveling through a solid barrier:
- The vapor molecules must reach the first barrier interface
- The permeant is absorbed at the first barrier interface
- Diffusion occurs through the solid barrier
- The permeant then undergoes desorption at the second barrier interface
- The vapor molecules diffuse away from the barrier
The processes in steps 2 and 4 are mainly determined by solubility of the permeant to the barrier material, while the diffusion coefficient determines step 3.
Materials for Glove Box Construction
Stainless Steel
Stainless steel is a widely used barrier material due to its low permeability to gases and water vapor. Its high density and minimal free volume between atoms make it an excellent barrier. Being robust, durable, and resistant to chemical corrosion means it is suitable for long-term use in various experimental conditions. For applications requiring minimal contamination risk, stainless steel glove boxes are ideal.

Image Credit: Ossila Ltd
Glass
The non-porous nature of glass makes it nearly impermeable to moisture and oxygen. Glass also allows researchers to monitor their work without compromising the sealed environment. Some glove boxes are made entirely of glass to allow the user full visibility into the working environment. However, as glass can be fragile, it is often combined with stainless steel to enhance durability and maintain low permeability.
Polymers
Polymers are transparent, cost-effective, and offer versatility in design and application. However, they are generally more permeable than metals and glass, which can pose a challenge in maintaining low oxygen and moisture levels. The permeability of polymers depends on their molecular structure and the state of the polymer—whether it is glassy, crystalline, or rubbery.
- Glassy polymers, such as PMMA or acrylic, have a glass transition temperature (Tg) higher than room temperature, making them rigid and brittle. They are less permeable than rubbery polymers but, due to non-equilibrium free volume pockets, still allow some moisture and oxygen ingress.1,2
- Rubbery polymers are more flexible and elastic because their Tg is lower than room temperature. The increased mobility of polymer chains creates a higher free volume, facilitating greater permeability to gases and moisture. Despite this, they can be a good choice for temporarily shielding samples when working outside of the lab.
- Crystalline polymers, such as polycarbonate or PTFE, offer a better packing density than their glassy or rubbery counterparts, resulting in lower permeability. However, imperfections at the grain boundaries can provide pathways for some ingress, though this is typically minimal compared to other polymers.3

Image Credit: Ossila Ltd
Choosing the Right Glove Box Material for Your Application
The right materials for your glove box should align with the specific demands of your application. For experiments where even trace levels of oxygen and moisture can be detrimental, like handling air-sensitive compounds or biological samples, stainless steel and glass are the best choices.
High-quality polymers can be used for less stringent applications or where budget constraints are a concern. Polymers, while more versatile and cost-effective, require careful consideration of their molecular structure and state to be effective in such applications. If you are choosing a polymer glove box, it may be best to choose a semi-crystalline polymer as they have the lowest levels of ingression.
Ultimately, the material must balance permeability, durability, and cost to ensure optimal performance in your lab setting.
References
- Glassy Polymer, J. C. Jansen, Encycl. Membr., 1 (2015); DOI: 10.1007/978-3-642-40872-4_270-1.
- Comparison Of Transport Properties Of Rubbery And Glassy Polymers And The Relevance To The Upper Bound Relationship, L. M. Robeson et al., J. Memb. Sci., 476 421–431 (2015); DOI: 10.1016/j.memsci.2014.11.058.
- Atom Movement In Materials, D. R. Askeland, Sci. Eng. Mater., 111–137 (1996); DOI: 10.1007/978-1-4899-2895-5_5.

This information has been sourced, reviewed and adapted from materials provided by Ossila Ltd.
For more information on this source, please visit Ossila Ltd.