Sponsored by SemplorReviewed by Louis CastelDec 19 2024
Lithium Cobalt Oxide (LiCoO2), also known as Lithium cobaltite, is a fundamental material in developing lithium-ion batteries. These batteries are used mainly as power sources for communication, computers, and consumer electronics.
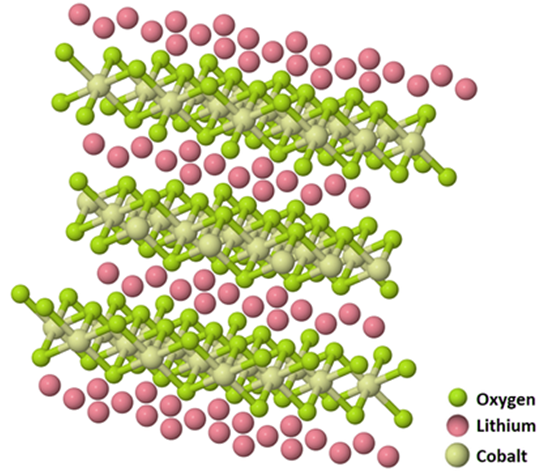
Figure 1. Schematic layered LiCoO₂ structure. Image Credit: Ossila
Originally proposed as an alternative to lithium metal cathodes, LiCoO2 provides improved stability and capacity, crucial for the needs of contemporary electronic devices.
This material’s continual development and frequent application highlight its value in the industry, fueled by its high volumetric and gravimetric energy density and impressive operational potential.
LiCoO₂ is categorized by its layered structure, in which cobalt atoms are located between oxygen layers in a trivalent oxidation state (Co3+). Lithium cations (Li+) reside between these cobalt-oxygen sheets, facilitating lithium ion movement, which is fundamental for battery operation.
The material is extremely useful for energy storage solutions with an elevated theoretical specific capacity of 274 mAh/g and an elevated discharge voltage of around 4.2 V versus Li+/Li.
Challenges and Current Applications
Despite its benefits, LiCoO2 faces difficulties relating to capacity retention and structural stability throughout battery operation. When half of the Li⁺ ions are removed during charging at approximately 4.0 to 4.2 V, a significant shift typically occurs in configurational entropy and structural rearrangement.
This alteration frequently reduces practical capacity by approximately 165 mAh/g, markedly lower than its theoretical capacity. Understanding and mitigating these impacts is critical for improving the performance and lifespan of batteries using LiCoO2.
Due to its simple processing and unparalleled energy density, LiCoO2 is the dominant battery material in the battery sector for portable electronics. However, its particle size and distribution nuances significantly affect general battery performance, dictating ion transport efficiency and capacity fading rate.
Microscopic examination of LiCoO2 using technologies such as scanning electron microscopy (SEM) is thus invaluable in advancing battery technology. The NANOS tabletop SEM performs exceptionally well for this application.
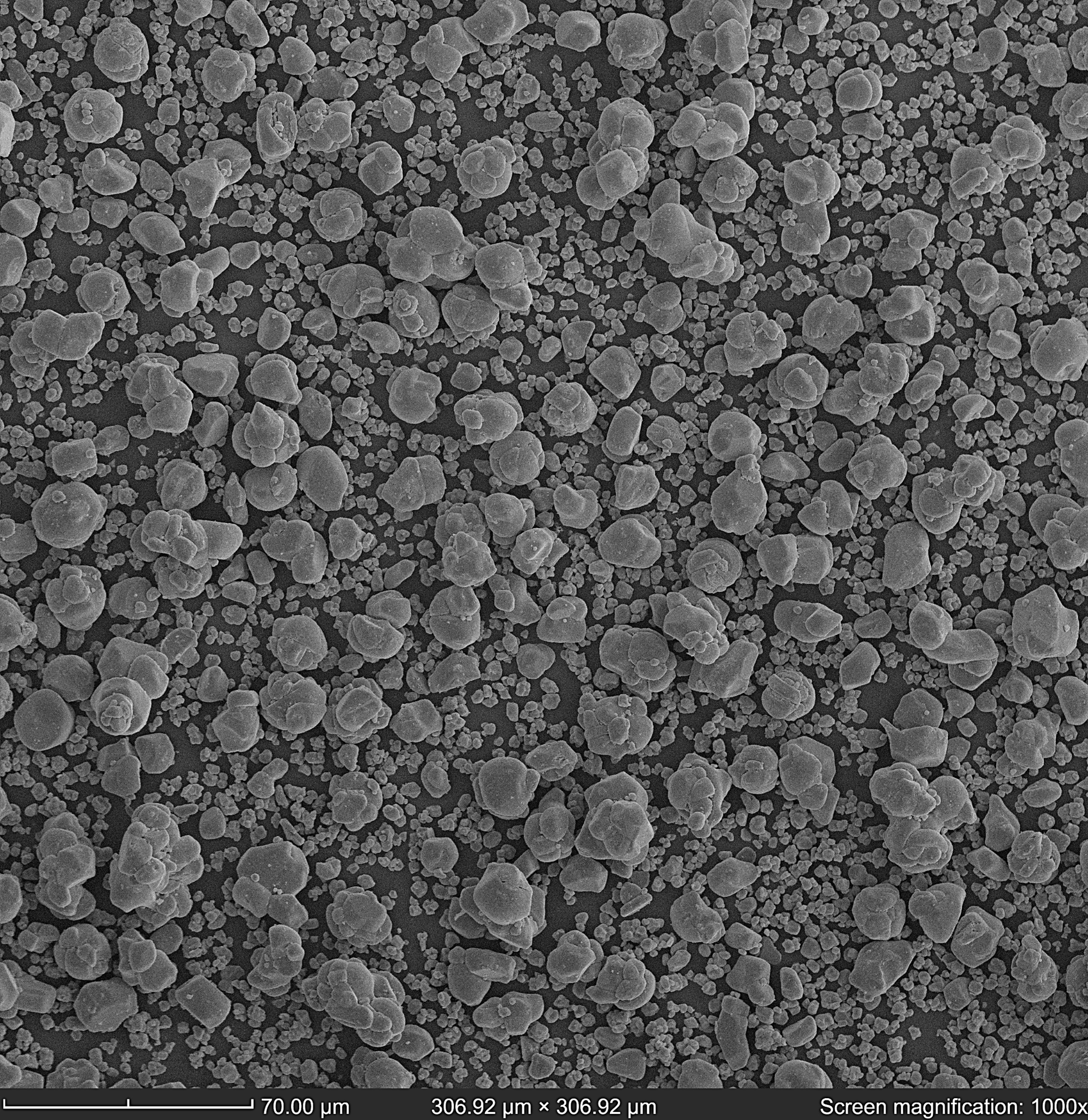
Figure 2. BSD image of LiCoO₂ particles. Image Credit: Semplor

Figure 3. BSD image of LiCoO₂ particle. Image Credit: Semplor

Figure 4. BSD image of smaller LiCoO₂ particles. Image Credit: Semplor
As displayed in figures 2-4, the particle size of the LiCoO2 is predominantly 10 µm, among smaller particles of 2-3 µm.
A predominant particle size (10 µm) indicates a somewhat uniform distribution of particle size, which is beneficial for battery performance. Particles of uniform size can result in more consistent packing density, electrode porosity, and ion transport properties within the battery.
Smaller particles (2-3 µm) scattered among larger particles can improve battery rate capability. Smaller particles offer a bigger surface area relative to their volume, which can accelerate lithium-ion diffusion rates. This is especially advantageous during high-rate charging and discharging, as it permits faster access to the lithium ions.
Larger particles, such as the 10-µm ones observed, usually experience less stress during lithium intercalation and deintercalation, which might decrease the probability of mechanical degradation and cracking compared to smaller particles. This could enhance long-term battery cycle stability.