Bioceramics in In Situ Radiotherapy One of the most common approaches in cancer treatment is the removal of the diseased parts, however unfortunately recovery or return of full function is seldom achieved. Non-invasive treatment techniques where only the cancer cells are destroyed were introduced in mid 80’s. In 1987, microspheres of 17Y2O3-19Al2O3-64SiO2 (mol%) glass, 20-30 µm in diameter were shown to be effective for in situ radiotherapy of liver cancer. 89Yttrium in this glass is non-radioactive but can be activated by neutron bombardment, to 90Y, which is a β-emitter with half life of 64.1 h. They are usually injected into diseased liver through the hepatic artery, and entrapped in small blood vessels, which block the blood supply to the cancer and directly irradiate the cancer with β-rays. Since the β-ray transmits living tissue only 2.5 mm in diameter and the glass microspheres have high chemical durability, the surrounding normal tissue is hardly damaged by the β-rays. These glass microspheres are already clinically used in Australia, Canada and U.S.A. The content of Y2O3 in the microsphere is, however, limited to only 17 mole%, as they are prepared by conventional glass melting techniques. Recently, Kokubo et al. successfully prepared pure Y2O3 polycrystalline microspheres 20 to 30 µm in diameter by high-frequency induction thermal plasma melting technique, (Figure 1). It was reported that they observed higher chemical durability than the Y2O3-containing glass microspheres. It was further reported that these ceramic microspheres are more effective for in situ radiotherapy of cancer. | 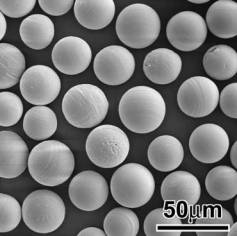
| | Figure 1. SEM image of Y2O3 microspheres for radiotherapy applications. | Ferrimagnetic and Ferromagnetic Materials in Cancer Treatment Oxygen is known to be poorly supplied to cancerous cells to produce lactic acid, and hence can be destroyed around 43ºC, whereas the normal living cells can be kept alive even around 48ºC. If ferri- or ferromagnetic materials are implanted around cancers and placed under an alternating magnetic field, it is expected that cancer cells locally heated can be destroyed by magnetic hysteresis loss of the ferri- or ferromagnetic materials. Kokubo and co-workers prepared a ferromagnetic glass-ceramic containing 36 wt% of magnetite (Fe3O4) 200 nm in size in a CaO-SiO2 matrix. It was reported that cancerous cells in medullary canal of rabbit tibia were completely destroyed when this glass-ceramic is inserted into the tibia and placed under an alternating magnetic field. This kind of invasive treatment, however, cannot be applied to humans, since cancer cells metastasize. In the case of humans, ferri- or ferromagnetic material must be injected to the cancer in a form of microsphere 20 to 30 µm in diameter through blood vessels similar to the radioactive microspheres. For this purpose, heat-generating efficiency of the ferrimagnetic material must be further increased. Recently microspheres of 20 to 30 µm in diameter in which magnetite particles 50 nm in size deposited on silica microspheres 12 µm in diameter, through deposition of β-FeOOH in a solution and its subsequent transformation into Fe3O4 at 600ºC under CO2-H2 gas atmosphere. It was reported that its heat generating efficiency was about four times that of the glass-ceramic described above. |
| A complete set of references can be found by referring to the original paper. Primary author: G. Heness and B. Ben-Nissan Source: Abstracted from “Innovative Bioceramics” in Materials Forum, Vol. 27, 2004. For more information on this source please visit The Institute of Materials Engineering Australasia. |