Sponsored by HORIBAAug 15 2005
Raman scattering and Fluorescence emission are two competing phenomena, which have similar origins. Generally, a laser photon bounces off a molecule and looses a certain amount of energy that allows the molecule to vibrate (Stokes process). The scattered photon is therefore less energetic and the associated light exhibits a frequency shift. The various frequency shifts associated with different molecular vibrations give rise to a spectrum that is characteristic of a specific compound.
In contrast, fluorescence or luminescence emission follows an absorption process. For a better understanding, one can refer to the diagram below.
Raman Scattering
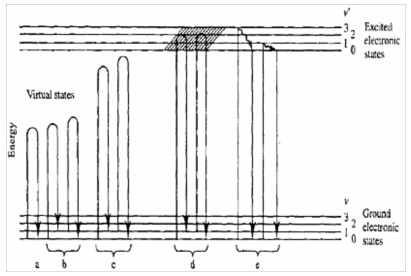
Figure 1. Mechanisms of various lightscattering processes. (a) Rayleigh, (b) nonresonance Raman, (c) pre-resonance Raman, (d) resonance Raman resonance fluorescence and (e) relaxed fluorescence.
Virtual states have to be considered to explain Raman scattering. This is related to the fact that the interaction of the photon with the molecule and the re-emission of the scattered photon occur almost simultaneously.
The existence of such virtual states also explains why the non-resonance Raman effect does not depend on the wavelength of the excitation, since no real states are involved in this interaction mechanism. In fact, the Raman spectrum generally does not depend on the laser excitation.
Resonance Fluorescence
However, when the energy of the excitation photon gets close to the transition energy between two electronic states, one then deals with resonance Raman or resonance fluorescence (fig.1, case (d)). The basic difference between these two processes is related to the time scales involved, as well as with the nature of the so-called intermediate states. In contrast with resonant fluorescent, relaxed fluorescence results from the emission of a photon from the lowest vibrational level of an excited electronic state, following a direct absorption of the photon and relaxation of the molecule from its vibrationally excited level of the electronic state back to the lowest vibrational level of the electronic state. A fluorescence process typically requires more than 10-9 s. In contrary, a Raman transition is completed within a picosecond or less.
It clearly appears that, depending on the laser wavelength, resonance effects (Raman or fluorescence), may or may not exist. If the excitation photon does not provide sufficient energy to the molecule, the required transition to generate fluorescence will not take place. However, if fluorescence is generated, it is often much more intense than Raman scattering, hiding Raman features. But because the Raman spectrum tends to be more informative than fluorescence, the Raman spectroscopist is continually searching for methods to avoid fluorescence.
Laser Excitation Wavelength
One method to avoid fluorescence emission is to select the laser excitation wavelength. For most examples, the choice of a near IR (NIR) or UV laser wavelength can avoid exciting fluorescence. In the first case, the laser photon does not have enough energy to excite molecular fluorescence. In the second case, the fluorescence may be excited, but the emission is widely separated in energy from the Raman signal so that the Raman spectrum can be recorded without the fluorescence interference.
A few examples demonstrating the effect of tuning the excitation wavelength on the fluorescence emission are shown below. These highlight the prime importance of a careful laser wavelength selection for each of these samples.

Figure 2. Polluted polymer.
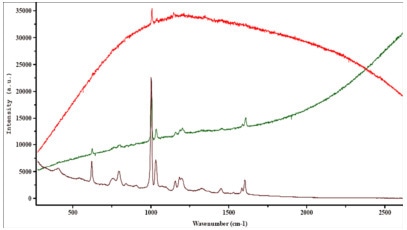
Figure 3. Pigment.
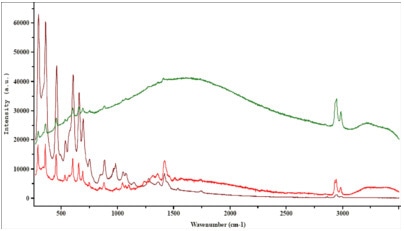
Figure 4. Industrial latex.
Sometimes, fluorescence may come from impurities of a polluted sample or from the matrix surrounding an inclusion. In these cases, use of a Raman microprobe on solid samples, can avoid or minimize background fluorescence by limiting the collection volume from which the Raman signal is acquired.
This can be achieved using the selective capability of the true confocal configuration of the Raman microscope. By closing the confocal hole, one indeed can define a smaller collection volume.
Conclusion
Although intense fluorescence emission sometimes renders the acquisition of useable Raman spectra very difficult, several ways to counteract it exist. Raman instruments offering the possibility to select the laser wavelength have a great in cases where samples show fluorescence in the visible range.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.