Introduction The syntheses of materials with various nanoscale spaces are currently under intensive investigation. Since carbon nanotubes, which have novel properties unlike those of either graphite or fullerene, were discovered by Iijima [1], various syntheses of micro- and nanotubes of TiO2 have been attempted by various methods such as template methods [2-4]. Recently, Kasuga et al. [5] treated TiO2 in the 10 M NaOH aqueous solution for 20 h at 383 K without the need for molds for replication or template and nanotubes with 8 nm in diameter and 100 nm in length were obtained. TiO2 nanotubes obtained by this chemical process are particularly interesting, because of their large specific surface area caused by nanotubular morphology, leading to the development high photocatalytic activities. This simple and low-cost synthetic method through chemical process may be applied in the fabrication of other oxide nanotubes. After their reports, many groups have investigated about their structure, formation mechanism, or synthetic condition for the nanotuluar products [6-9]. Recently, the products with various morphologies were obtained by modifying synthetic conditions for this hydrothermal process. Nanowires were obtained by hydrothermal process from anatase and successively post heat-treatment [10]. Yuan and co-workers found that the nanofiber was successfully prepared when anatase was prepared by conventional hydrothermal treatments at 523 K [11]. In this study, a conventional hydrothermal method was used to synthesize nanotubular products. As a starting material, various TiO2 sources are available for the synthesis of nanotubes. In this study, special, anatase-type TiO2 powders were conventionally hydrothermal-treated in 10 M NaOH aqueous solution at 383 to 473 K. The structural investigations of products obtained by various synthetic conditions were analyzed by various methods, such as X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray absorption near edge structure (XANES). Experimental Procedure Synthesis of Samples As a starting material, two grams of anatase-type TiO2 (Kojundo Chem., Japan) were used. They were added to aqueous 10 M NaOH solution (15 ml). Then, the specimens were treated under conventional hydrothermal reactions at 383 to 423 K for 48 to 96 h. Products obtained after hydrothermal treatments were washed sufficiently with deionized water, filtered, and dried at 323 K. Characterizations Crystalline phase of samples were determined by XRD (Rint 2500, Rigaku Co., Ltd, Japan) using CuKα radiation at 40 kV and 50 mA. The XRD profiles were collected between 5-60°of 2θ angles with a step interval of 0.01° and scanning rate of 4۫/min. Various microstructural analyses were performed by SEM (S-800, Hitachi, Japan) with accelerating voltage of 20 kV and TEM (JEM2010/SP, JEOL) with accelerating voltage of 200 kV. The Ti K-edge (XANES) was recorded at room temperature at BL01B1 in SPring 8, Japan synchrotron radiation facility. The operating conditions in the storage ring were at energies of 8.00 GeV and intensities of about 150 mV. The Ti K-edge XANES data for the study was corrected by transmission mode using the two-crystal Si (111) monochromator. Ti metallic foil was used to carry out for the energy calibration. The energy was scanned by 0.25 eV steps over the energy range for the Ti-K edge XANES. XANES analyses were carried out by subtracting a linear background computed by a least-square fitting from the pre-edge region and normalized. Results and Discussion Figures 1a and 1b show SEM and TEM images of the product prepared by a hydrothermal treatment of anatase-type TiO2 powders with 10 M-NaOH aqueous solution for 96 h at 383 K. From these SEM observations, it was found that the product obtained by hydrothermal treatments had a nanowhisker-like morphology. According to the results of TEM observations, these nanowhisker-shaped products had nanotubular structures with approximately 10 nm in outer diameter and approximately 5 nm in inner diameter (as shown in Figure 1b) as well as those reported by Kasuga et al. [5]. Also, the results of electron diffraction indicated that these products were crystalline(not shown in Figure 1). The XRD patterns of these nanotubular product and anatase-type TiO2 are shown in Figure 2. XRD analysis showed that the nanotubular products by hydrothermal treatments for 96 h at 383 K had a single phase, although the nanotubular products by hydrothermal treatments for 6 h to 32 h at 383 K contained a mixture of unreacted anatase-type TiO2 and nanotubes. Consequently, from these XRD results, nanotubes prepared by hydrothermal treatment for 96 h could be identified as H2TinO2n+1 type phase. 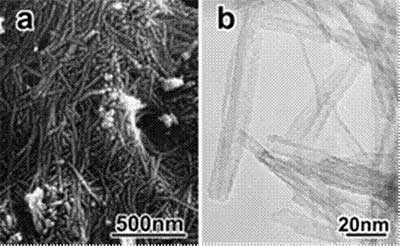
Figure 1. (a) SEM image and (b) TEM image of the products prepared by a hydrothermal treatment of anatase-type TiO2 for 96 h at 383 K. No unreacted anatase-type TiO2 existed in the products after hydrothermal treatments for 96 h, showing that the hydrothermal reaction for these nanotubes was completely finished after 96 h. 
Figure 2. XRD patterns of (a) anatase-type TiO2 as a starting material and (b) the products prepared by a hydrothermal treatment of anatase-type TiO2 for 96 h at 383 K. Triangle marks shows the products prepared by the hydrothermal treatments. Background subtracted and normalized Ti K-edge XANES for anatase-type TiO2 and nanotubular product prepared by static hydrothermal process are shown in Figure 3. As shown in Figure 3, the position of the absorption edge of Ti K-edges obtained for nanotube and anatase were located in the same position. The XANES results imply that Ti ions in titanate nanotubes are Ti (IV). 
Figure 3. Ti K-edge XANES of (a) anatase-type TiO2 as a starting material and (b) the products prepared by hydrothermal treatment of one for 96 h at 383 K. Pre-edge of Ti K-edge XANES spectra are widely used to derive information on the coordination environment of Ti (IV) in structurally complex oxide materials, such as titanosilicate glasses etc [12-14]. Pre-edge of Ti K-edge XANES for anatase-type TiO2, nanotubular product prepared by this hydrothermal process, and layered Na2Ti3O7 are shown in Figure 4. As shown in Figure 4, the pre-edge features of the nanotube were significantly similar to ones of titanates such as Na2Ti3O7. Therefore, it was considered that the nanotubes prepared by this hydrothermal process could be composed of the titanate compound. Here, although the peak is located at 4963.8 eV for the nanotube in pre-edge of Ti-K edge XANES, the peak is not consistent with ones for Na2Ti3O7. Since this peak at 4963.8 eV is also observed for anatase-type TiO2, it was considered that the peak was derived from the anatase structure. Other reports also showed the peak around 4963.8 eV for anatase-type TiO2. The peak around 4963.8 eV from anatase structure resulted from nanotubular structure, not anatase-type TiO2 as a starting material, since XRD result showed nanotubular products had no peaks from anatase-type TiO2 (as shown in Figure 2). In other words, it was considered that titanate nanotube partly had anatase-like local structure on the formation of the nanotubular structures. 
Figure 4. Pre-edge of Ti K-edge XANES for (a) anatase-type TiO2, (b) the product prepared by a hydrothermal treatment of anatase-type TiO2 for 96 h at 383 K, and (c) Na2Ti3O7 (for reference). Conclusions H2TinO2n+1 products with nanotubular morphologies were synthesized by the hydrothermal method for anatase-type TiO2 in NaOH aqueous solution systems at low temperatures and for short time in this study. The nanotubular product with approximately 10 nm in outer diameter and approximately 5 nm in inner diameter was obtained by static hydrothermal treatment of anatase-type TiO2. From TEM images and pre-edge of Ti K-edge XANES spectra, it was found that the nanotubes prepared by this hydrothermal process may be composed of a titanate compound and also partly had anatase-like local structure on the formation of the nanotubular structures. The structural evaluation for these nanotubes would lead to the clarification of the mechanism for synthesis of nanotubes and development of useful nanotubular materials for several applications. Acknowledgements X-ray absorption fine structure (XAFS) measurements of Ti K-edge were carried out at BL01B1 in SPring8. Authors greatly thank for the technical support and discussion from Japan Synchrotron Radiation Research Institute (JASRI) (2004B0166-NXa-np). References 1. S. Iijima, “Helical microtubules of graphitic carbon”, Nature, 354 (1991) 56-58. 2. P. M. Ajayan, O. Stephan, P. Redlich and C. Colliex, “Carbon nanotubes as removable templates for metal oxide nanocomposites and nanostructures”, Nature, 375 (1995) 564-567. 3. P. Hoyer, “Formation of a Titanium Dioxide Nanotube Array”, Langmuir, 12 (1996) 1411-1413. 4. H. Imai, Y. Takei, K. Shimizu, M. Matsuda and H. Hirashima, “Direct preparation of anatase TiO2 nanotubes in porous alumina membranes”, J. Mater. Chem., 9 (1999) 2971-2972. 5. T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino and K. Niihara, “Formation of titanium oxide nanotube”, Langmuir, 14 (1998) 3160-3163. 6. Q. Chen, G. H. Du, S. Zhang and L. M. Peng, “The structure of trititanate nanotubes”, Acta Crystallogr. B, 58 (2002) 587-593. 7. Q. Chen, W. Zhou, G. Du and L. M. Peng, “Trititanate nanotubes made via a single alkali treatment”, Adv. Mater., 14, (2002) 1208-1211. 8. R. Ma, Y. Bando and T. Sasaki, “Nanotubes of lepidocrocite titanates”, Chem. Phys. Lett., 380 (2003) 577-582. 9. Nakahira, W. Kato, M. Tamai, T. Isshiki, K. Nishio and H. Aritani, “Synthesis of nanotube from a layered H2Ti4O9 H2O in a hydrothermal treatment using various titania sources”, J. Mater. Sci., 39 (2004) 4239-4245. 10. M. Zhang, Z. S. Jin, J. J. Yung and Z. J. Zhang, “Effect of annealing temperature on morphology, structure and photocatalytic behavior of nanotubed H2Ti2O4(OH)2”, J. Molec. Catal. A: Chem., 217 (2004) 203-210. 11. Z. Y. Yung and B. L. Su, “Titanium oxide nanotubes, nanofiber and nanowires”, Colloids Surf. A, 241 (2004) 173-183. 12. T. Blasco, M. A. Camblor, A. Corma, P. Esteve, J. M. Guil, A. Martinez, J. A. Perdigon and S. Valencia, “The state of Ti titanoaluminosilicates isomorphous with Zeolite β”, J. Am. Chem. Soc., 115 (1993) 11806-11813. 13. F. Farges, G. E. Brown and Rehr, “Coordination chemistry of Ti (IV) in silicate glasses and melts: I. XAFS study titanium coordination in oxide model compounds”, Geochim. Cosmochim. Acta, 60 (1996) 3032-3053. 14. G. Mountjoy, D. M. Pickup, G. W. Wallidge, R. Anderson, J. M. Cole, R. J. Newport and M. E. Smith, “XANES study of Ti coordination in heat-treated (TiO2)x(SiO2)1-x xerogels”, Chem. Mater., 11 (1999) 1253-1258. Contact Details |