Handheld Thermo Scientific Niton energy-dispersive x-ray fluorescence (EDXRF) analyzers, commonly known as XRF analyzers, are able to quickly and nondestructively determine the elemental composition of:
- Metal and precious metal samples
- Rocks and soil
- Slurries and liquid samples
- Painted surfaces, including wood, concrete, plaster, drywall and other building materials
- Dust collected on wipe samples
- Airborne heavy elements collected on filters
Elemental Analysis
Up to 30 or more elements may be quantified simultaneously by measuring the characteristic fluorescence x-rays emitted by a sample. Niton XRF analyzers can quantify elements ranging from magnesium (element 12 in the periodic table) through plutonium (element 94), measuring fluorescent x-ray energies from twelve hundred fifty electron volts (1.25 keV) up to 100 keV. Niton Analyzers also measure the elastic (Raleigh) and inelastic (Compton) scatter x-rays emitted by the sample during each measurement to determine, among other things, the approximate density and percentage of the light elements in the sample.
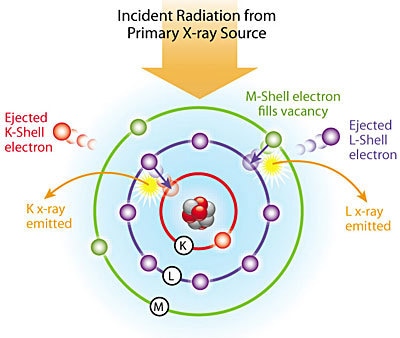
Figure 1. XRF Excitation Model
How Does EDXRF Work
How does EDXRF work? Each of the atomic elements present in a sample produces a unique set of characteristic x-rays that is a fingerprint for that specific element. EDXRF analyzers determine the chemistry of a sample by measuring the spectrum of the characteristic x-rays emitted by the different elements in the sample when it is illuminated by high energy photons (x-rays or gamma rays). These photons are emitted either from a miniaturized x-ray tube, or from a small, sealed capsule of radioactive material.
A fluorescent x-ray is created when a photon of sufficient energy strikes an atom in the sample, dislodging an electron from one of the atom's inner orbital shells (lower quantum energy states). The atom regains stability, filling the vacancy left in the inner orbital shell with an electron from one of the atom's higher quantum energy orbital shells. The electron drops to the lower energy state by releasing a fluorescent x-ray, and the energy of this fluorescent x-ray (typically measured in electron volts, equal to the specific difference in energy between two quantum states of the dropping electron.
Quantum States
Because the quantum states of each electron orbital shell in each different type of atom (each of the atomic elements) is different, the energies of the fluorescent x-rays produced by different elements are also different: When a sample is measured via XRF, each element present in the sample emits its own unique fluorescent x-ray energy spectrum. By inducing and measuring a wide spectrum of the range of different characteristic fluorescent x-rays emitted by the different elements in the sample, handheld Niton XRF analyzers rapidly determine the elements present in the sample and their relative concentrations- in other words, the elemental chemistry of the sample. For samples with known ranges of chemical composition, such as common grades of metal alloys, a Niton Analyzer also identifies most sample types by name, typically in seconds.

This information has been sourced, reviewed and adapted from materials provided by Thermo Fisher Scientific.
For more information on this source, please visit Thermo Scientific Portable Analyzers for Material ID.