Malvern Panalytical is a leading supplier of analytical solutions for particle characterization and rheological applications. Advanced measurement technologies are combined with robust mechanical designs and comprehensive data handling and automation software, to provide systems that are relevant across a wide range of industrial and fundamental research applications.
Material characterization data such as size distribution, particle shape, zeta potential, molecular weight, and bulk material properties, can be accurately and reproducibly measured using instruments from the Malvern Panalytical range. Technologies used include laser diffraction, image analysis, laser Doppler electrophoresis, static and dynamic light scattering, capillary rheometry and strain controlled and stress controlled rheometry.
Disperse Systems and Materials in Use Today
Many materials in use today are disperse systems where one substance (often particulate) is dispersed in another phase. These material types include adhesives, agrochemicals, cement, ceramics, colloids, cosmetics and personal care formulations, food and drink, mining and mineral slurries, paints, inks and surface coatings, pharmaceuticals and polymer systems.
Rheology and Particle Properties in the Ink Industry
In the inks industry, the understanding of rheology and particle properties allows solid pigment content to be changed in different formulations whilst maintaining the critical rheological characteristics required for optimized printing.
Rheology and Particle Properties in the Cement Industry
In the cement industry, the understanding of rheology and particle properties, such as the aggregate morphology, allows flow behaviour during processing and application to be controlled.
Rheology and Particle Properties in the Cosmetic and Personal Care Industry
In the Cosmetics and Personal Care industries, it is essential to understand the relationship between rheology and particle properties to provide the optimum balance in terms of formulation, consumer acceptance and application performance.
Rheology and Properties of Dispersed Particles
The physical properties of the dispersed particles, such as the average particle size, the size distribution, the zeta potential or charge on the particles and even the shape of the particles all help influence the overall (bulk) materials properties such as the rheology.
This “Ten Ways” guides you through some of the fundamental properties of the dispersed system, and demonstrates how these affect the rheological properties. Whereas it in interesting to understand the bulk material properties, such as rheology, which are associated with the changes in the particle size, shape and zeta potential, these examples will also demonstrate that this understanding allows the rheology of the material to be controlled.
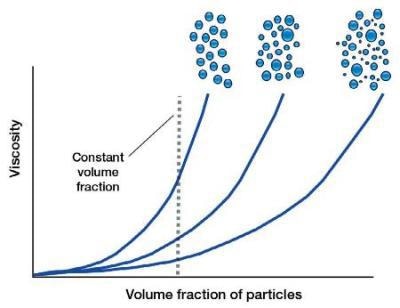
Increasing Viscosity and Decreasing Particle Size
For a constant volume fraction, when the particle size is decreased, the number of particles increases. Therefore, the number of particle-particle interactions increases, so the viscosity of a sample typically increases. As particle-particle interactions are weak forces, the effect is seen more at low shear rates.
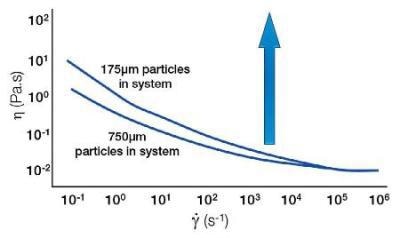
Decreasing Viscosity and Increasing Particle Size
Conversely, if the particle size is increased, this leads to a smaller number of particle-particle interactions. Again, due to the weak nature of this association, the effect is seen most dramatically at low shears.
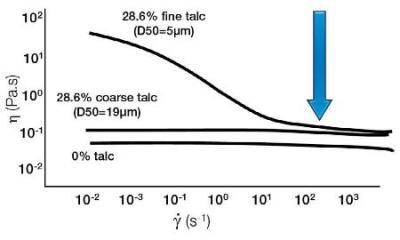
Particle Size Distribution and Viscosity
Particles which have a wide span / distribution (large polydisperity) tend to pack better than a system of particles of all the same size (a narrow distribution). This basically means that a wide distribution of particles has more free space to move around, which therefore means it is easier for the sample to flow, i.e. a lower viscosity. So, tightening up the particle distribution can increase the stability of a system.
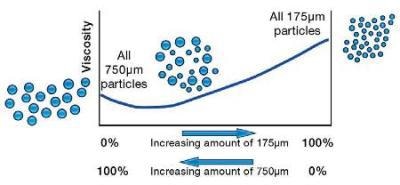
Particle Size and Distribution and Viscosity
For example, on keeping the volume fraction the same, a sample of relatively large particles with a small proportion of small particles will have a viscosity lower than either the small particles or large particles alone.
This is basically due to the two competing effects of changing the number of particle-particle interactions on changing the size and also changing the polydispersity. Both affect the viscosity, however, in this case, the effect of polydispersity dominates at one particular ratio.
Number of Particles and Flow Behavior
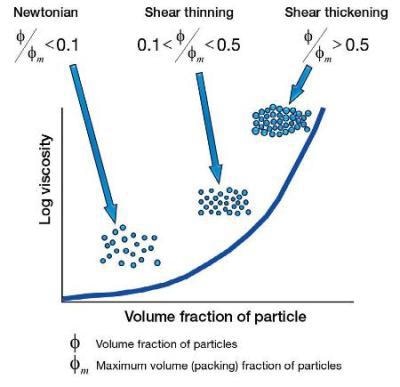
Ensuring the particle size is constant, where more and more particles are introduced the flow behavior will generally go from Newtonian (so few particles that they do not interact with each other), to shear thin (now the particles can interact, but the forces are so small that this interaction can be broken down with an increasing shear rate, therefore a shear thinning property), to shear thickening (where there are so many particles that on increasing the shear rate the particles now start to physically collide with each other which causes a shear thickening effect).
Zeta Potential and Behavior of Sub-Micron Particles
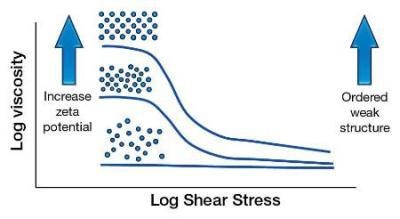
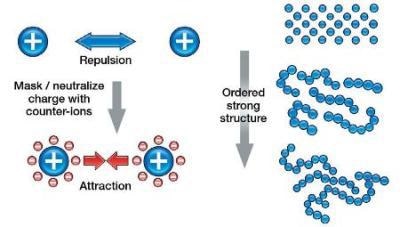
As the zeta potential is increased, the particles are forced to stay away from each other. This is basically preventing the particles from flowing freely, hence the viscosity increases. The effect is seen more at lower shear rates as this is a small force.
Zeta Potential and Behavior of Particles Greater than a Micron
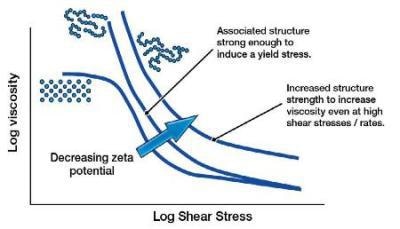
For larger particles, the settling force of gravity on the particles will overcome any repulsion of the particles due to the electrostatic charge / zeta potential. However, as these large particles can no longer fully aggregate (hydration layer), this close range, yet strong, Van der Waals attractive force can increase the low shear viscosity.
Smooth Particles and Shear Viscosity
Smooth particles have a resistance to move as there is typically an association between the different particles, which is often a chemical interaction. However, with non-smooth particles there will also be a mechanical resistance, and also the chemical association can be increased. Therefore, non-smooth particles have higher low shear viscosity and a higher yield stress.
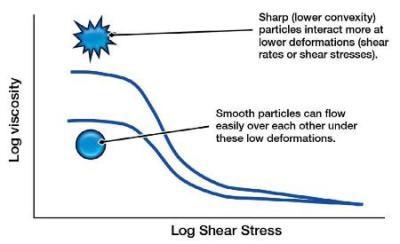
The Effect of Particle Morphology on Shear Viscosity
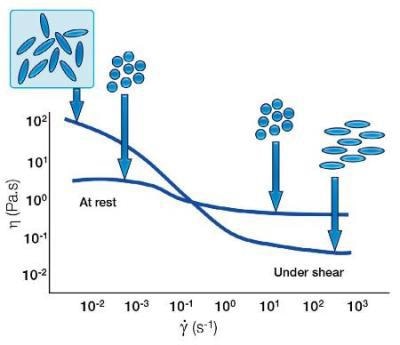
With spherical particles, there are typically particle-particle interactions which break down under shear to give a shear thinning behaviour. However, with elongated particles the random orientation leads to a higher barrier to start flow; an increase in low shear viscosity. However, under shear, these elongated particles can orient themselves to be streamlined with the direction of flow. They are therefore easier to flow, resulting in a lower shear viscosity than the spherical same size equivalent.
Shear Thinning Behavior of Particles
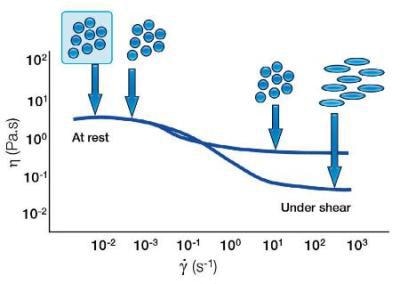
With soft particles, a forced shear can change the shape of the particle. This can lead to the particles elongating and aligning under shear resulting in a more shear thinning system.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.