Sponsored by HORIBASep 24 2012
The zeta potential of polyethylenimine, PEI, is measured as a function of polymer concentration with the SZ-100 Nanoparticle Analyzer. Zeta potential decreases as polyelectrolyte concentration is increased due to the increasing ionic strength of the suspension and intermolecular interactions. Charged polymers, that is, polyelectrolytes can be analyzed with the SZ-100.
Introduction
Polyelectrolytes are polymer chains with an electrolyte group on every repeat unit. These polymers are charged when dissolved in a polar solvent due to dissociation of small counterions that leave behind a charged macro ion. In addition to synthetic polymers, the topic of this note, polyeletrolytes include biologically important molecules such as polypeptides and DNA.
The charge on a polyelectrolyte is a function of the solution conditions. Unlike the case of simple electrolytes, not all counterions will dissociate away from a polyelectrolyte. As the magnitude of the charge on the chain increases, it becomes progressively more diffi cult to remove the next ion. This phenomenon is known as counterion condensation.
Polyelectrolyte behavior is different from that of uncharged polymers. For example, due to electrostatic repulsion between different segments on the same chain, a polyelectrolyte chain will tend to be more rod-like than a typical Gaussian chain. Furthermore, due to long range electrostatic interactions, polyelectrolyte solution properties are different from those of neutral polymers. For example, the second virial coeffi cient (a measure of solution thermodynamics) can be an order of magnitude higher for a polyelectrolyte than a neutral polymer. These effects will lead to signifi cant changes in solution viscosity.
Due to these solution effects polyelectrolytes have a number of applications. They are used as viscosity modifi ers. Furthermore, due to their charge, they are used to modify the surface charge of nanoparticles to either stabilize suspensions by increasing particle surface charge or to initiate fl occulation by a) neutralizing surface charge and b) acting as a bridge between particles.
Polyethylenimine (PEI) is a cationic polyelectrolyte. The structure is given in Figure 1. PEI is used to attach negatively charged cells in biology. It is also used for DNA transfection (introducing new DNA into cells). PEI can also be used to capture carbon dioxide. Much of the useful behavior of PEI is obtained by its charged state and the ability to manipulate it using pH.
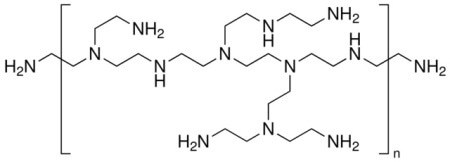
Figure 1. Illustration of structure of branched PEI. The NH groups give the polymer a charge that is manipulated via, among other things, solution pH.
Since they are large molecules, polyelectrolytes scatter light in much the same way as particles. Due to their charge, they respond like particles to an applied electric fi eld. Thus, one can measure polyelectrolyte mobility and extract a zeta potential value to characterize these materials.
Materials and Methods
Branched PEI was obtained from Sigma Aldrich as a 50 weight percent solution in water. This was then further diluted with 1 mM aqueous KCl to prepare solutions with appropriate polymer concentration. The small amount of KCl is used as a background electrolyte to ensure that the effect of small quanitites of impurities do not dramatically affect zeta pottential results. The polymer molecular weight was given by the manufacturer as 750000 g/mol by light scattering.
Zeta potential was then measured with the SZ-100, shown in Figure 2. Measurements were repeated six times.
Figure 2. SZ-100 Nanoparticle Analyzer.
Results and Discussion
The obtained zeta potential values are shown in Table 1 below. Figure 3 shows the effect of concentration on zeta potential. As expected, zeta potential decreases with increasing ionic strength due to the increasing concentration of macro-ions and increasing chain overlap.
Table 1. Zeta potential of PEI as a function of polymer concentration
| PEI concentration, mg/mL |
Avg. Zeta Potential, mV |
Standard Deviation, mV |
| 1.7 |
21.4 |
1.8 |
| 5.0 |
13.4 |
0.9 |
| 16.8 |
9.2 |
0.6 |
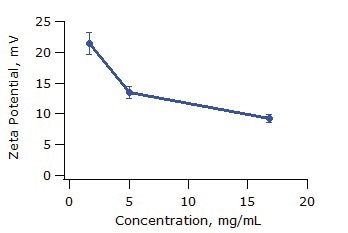
Figure 3. Zeta potential of PEI as a function of polymer concentration. Error bars correspond to one standard deviation.
Conclusions
The SZ-100 can be used to characterize the charge of polymeric species. Zeta potential decreases as polyelectrolyte concentration is increased due to the increasing ionic strength of the suspension and intermolecular interactions. The zeta potential of charged polymers, such as polyelectrolytes can easily be analyzed with the SZ-100.

This information has been sourced, reviewed and adapted from materials provided by HORIBA.
For more information on this source, please visit HORIBA.