The occurrence of aggregation in immunoglobulin biopharmaceuticals is a major issue during all stages of drug development. With a higher number of monoclonal antibody titers generated in bioreactors, finding techniques and processes for the effective aggregate removal becomes necessary for manufacturers.
Analysis of Aggregate Removal Capability of a Purification Procedure
Analytically, a high throughput aggregate detection technique would accelerate the evaluation and validation step in the implementation of the aggregate removal process. A purification procedure’s aggregate removal capability is evaluated by generating and passing an IgG feed with very high aggregate levels through a purification device in flow through mode under two various buffer conditions to analyze the feed, flow-through fractions, and regeneration samples.
The species population for sample set 1 acquired by SEC-MALS analysis is represented in Figure 1. From the observation, the composition of the feed sample was 45% high molecular weight aggregates (HMW), 39% monomer and 16% dimer. Monomer and dimer species were observed in the earlier fractions (fractions 1-6), while HMW aggregates became visible in the later fractions for the flow through samples.
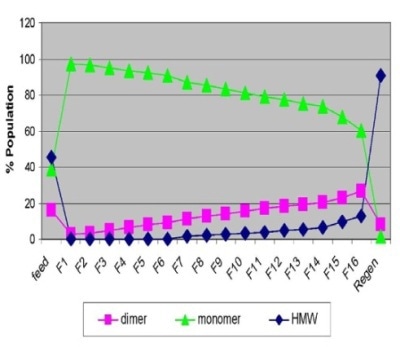
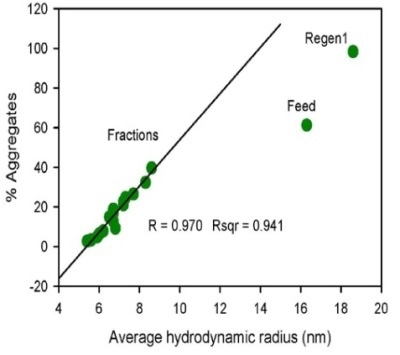
Figure 1. Flow through profile of monomer, dimer and HMW species obtained by SEC-MALS (upper left) and the correlation plot of percentage aggregates by SEC-MALS vs. average hydrodynamic radius measured by DLS (upper right).
Analysis Results
The regeneration peak comprised predominantly of HMW species, representing roughly 90% of the population. These results show that the breakthrough of non-monomer species was evident as early as fraction 1.
DLS detected a sudden increase in average hydrodynamic radius (Rh) between fraction 1 and fraction 2, which was in line with the aforementioned observations, as shown in Figure 2.
This revealed that the early breakthrough of non-monomer species was in fraction 2, if not in fraction 1.
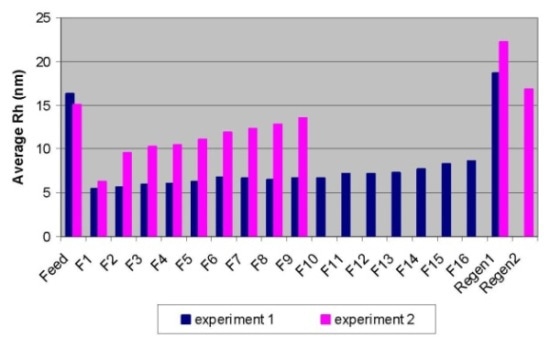
Figure 2. Breakthrough curves for Experiment 1 (in blue) and Experiment 2 (in pink) obtained by DLS.
The immediate increase in the average hydrodynamic radi between fraction 5 and fraction 6 also implied the immediate increase in aggregates (dimers or HMW species).
The regeneration sample’s measured Rh value is 18.6 nm, which is higher than the feed sample’s value of 16.3 nm, implying that the HMW population is higher than that for the feed sample. The plotting of the percentage of aggregates acquired by SEC-MALS versus the average Rh determined by DLS (Figure 1) found an excellent correlation for the flow through fractions.
Unlike SEC-MALS, DLS is not able to provide the species distribution, but it is a fast analytical instrument to determine the conditions that facilitate effective aggregate removal from monomeric IgG. As depicted in Figure 2, the operating condition in experiment 1 showed better separation when compared to condition 2.
Conclusion
The results showed that DLS was able to serve as a quick reliable analytical instrument to maximize the operating conditions for removal of aggregates.
The time required for the completion of the data acquisition for a set of 96 samples using SEC-MALS would be 32 hours excluding the time for sample preparation, HPLC set up and final data analysis.
On the other hand, the installation of DynaPro Dynamic Light Scattering (DLS) plate reader significantly reduced the data acquisition time and optimized the assay capabilities of Millipore.

This information has been sourced, reviewed and adapted from materials provided by Wyatt Technology.
For more information on this source, please visit Wyatt Technology.