Today, nasal spray products are being widely used for delivering both systemic and locally-acting therapies, especially migraine and hormone treatments. Nasal passages contain a rich blood supply, and are proximal to the central nervous system which holds significant benefit.
Particle Size
According to the regulatory guidance for nasal sprays, it is important to test the device and the formulation simultaneously because it is this combination which defines the special characteristics of the delivered dose.
The particle size of the delivered droplets strongly influences the success of drug delivery. As per the guidance, the size of the delivered droplet is a vital property that influences the nasal deposition of sprays and aerosols. For instance, extremely small droplets in the sub-10 micron range may be drawn into the lungs while those that are very large are likely to remain at the front of the nasal passages instead of depositing at the intended site.
Therefore, it is recommended that the size of the particle should be measured pre- and post-actuation in order to ensure that clinical efficacy requirements are fulfilled and that the active pharmaceutical ingredient (API) particles are not modified by the delivery process.
Nasal Spray Development
Standard nasal sprays often contain an API which is either suspended or dissolved in an aqueous medium. Patients can administer these products on their own through their nasal cavity. However, effective drug delivery depends on several factors such as the properties of the formulation, patient technique and physiology, and the properties of the spray pump. During the development of nasal spray, high importance is given to optimize the device and formulation to deliver strong performance for the target user group, which is likely to be very broad.
When it comes to the droplet size, the target range is usually between 20 and 120 µm. Droplets that fall in this size range are generally deposited beyond the nasal valve and thus maximize the therapeutic effect. However, particles less than 10 µm may be inhaled into the lungs and hence their presence should be assessed to rule out clinical risks related to pulmonary delivery of the API.
To this end, the task of a nasal spray developer is to understand and control the interactions between device and formulation and control the parameters that dictate performance for optimum clinical efficacy. By tuning some of the variables, such as pre-compression ratio, action of the pump, and the shape, length and orifice size of the actuator, product developers can customize nasal spray devices to deliver the required size of droplets for patient use.
Laser Diffraction and Microscopy Techniques
Laser diffraction is a non-destructive particle sizing technique and is suitable for measuring the size of a droplet ranging from 0.1 to 3000µm, while microscopy or imaging technique is generally used when studying suspended API.
This article examines the use of automated imaging and laser diffraction (Spraytec, Malvern Panalytical) combined with spectroscopic identification (Morphologi G3-ID, Malvern Panalytical) in the manufacture of nasal spray products.
The article also demonstrates how the two methods merge to provide a better understanding as well as the regulatory information needed to develop and improve nasal spray products in a cost-efficient manner.
Laser Diffraction
Laser diffraction can be used for characterizing dry as well as wet spray samples, and with minimal calibration requirements, it can be used to examine spray formation and dispersed particle size. This technique is used to ensure efficacy, safety and quality.
Through laser diffraction, the size of a particle is measured from the scattering pattern created as particles traverse via a collimated light beam. Larger particles produce a stronger signal at narrow angles, while smaller particles disperse light weakly at wide angles.
Laser diffraction analyzers sense the scattered light pattern created by a sample and apply the Mie theory of light to produce a complete particle size distribution from this pattern.
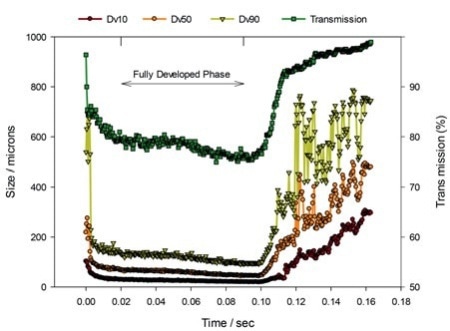
Figure 1. Laser diffraction data tracking the evolution of particle size, Dv10, Dv50 and Dv90, and transmission, a measure of droplet concentration, during a nasal spray event.
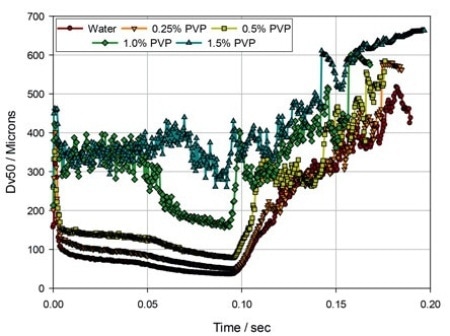
Figure 2. The evolution of droplet size (Dv50) during delivery via a nasal spray pump, for solutions of PVP in water.
Figure 1 illustrates standard nasal spray data measured utilizing a laser diffraction particle size analyzer configured for spray measurement (Spraytec, Malvern Panalytical). Figure 2 demonstrates some laser diffraction information from experimental studies performed to review the impact of formulation viscosity on delivered droplet size and atomization behavior.
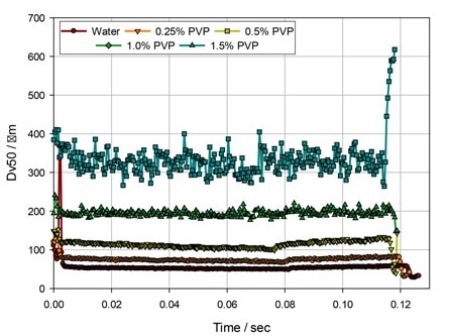
Figure 3. The evolution of droplet size (Dv50) during delivery with an Equadel (Valois Pharma) nasal spray pump, for solutions of PVP in water.
Figure 3 demonstrates data for the same PVP solutions atomized utilizing an Equadel pump (Aptar Pharma). These comparative data shows that varying the pump mechanism can be a successful strategy to obtain improved atomization with a more viscous formulation.
Automated Imaging
With recent advances in data analysis software and camera technology, automated imaging has benefited considerably over the last decade. Using automated imaging, the particle size of the API in a nasal spray formulation was determined before and after drug delivery to ascertain whether the atomization process via the nasal spray pump was causing any change.
Samples were suitably distributed on to a measurement plate and then studied to collect both shape and size data for the formulation.
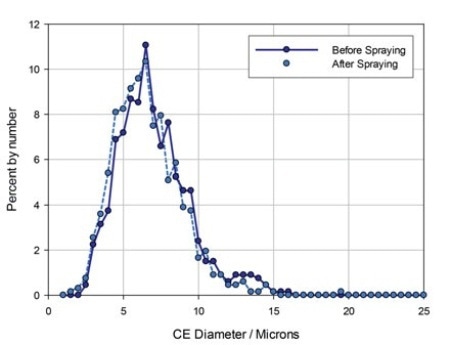
Figure 4. Particle size data for the API in a nasal spray formulation measured before and after spraying.
In this product, the API and excipient are differently shaped and can be easily distinguished by certain shape parameters. CE diameter values and particle size data were then produced just for these particles. The results are illustrated in figure 4.
Combination of Automated Imaging and Chemical Identification
In nasal spray studies, the parameters created by imaging can be utilized to classify particle populations to detect and obtain data for the API alone. However, microscopy and imaging have limitations in differentiating between excipients and API which are similar in shape and size.
This issue can be overcome by supplementing imaging with a chemical identification method such as a Raman spectroscopy. By gathering Raman spectra for particle populations of interest and linking them with reference spectra, one can identify API particles and collect information exclusively for them.
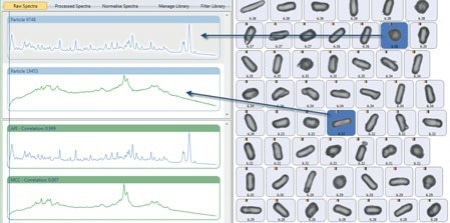
Figure 5. Two unique particles distinguished by the correlation of their Raman spectra with reference spectra. (shown in green)
Conclusion
Laser diffraction techniques provide droplet size data in real-time to understand the dynamics of atomization and supports the optimization of device as well as formulation.
Imaging techniques, when supplemented with spectroscopy, is a powerful tool that can be used to study nasal spray products. Together, these techniques support the fast and cost-effective development of nasal spray products and hold value in all aspects of nasal spray development.

This information has been sourced, reviewed and adapted from materials provided by Malvern Panalytical.
For more information on this source, please visit Malvern Panalytical.