Oct 16 2009
Mercury, the silvery liquid formerly used in thermometers, is now known to be highly toxic. The worst of the toxins are organic mercury compounds, such as methylmercury. Most previous analytical procedures for the detection of methylmercury were technically difficult and could only be carried out in a laboratory. A Spanish and German team has now developed a new approach to detect the poison rapidly, easily, and on the spot. As the scientists report in the journal Angewandte Chemie, it is possible to imagine a rapid test in which test strips are simply dipped into suspect samples.
Mercury enters the environment through slashing and burning of rain forests and the combustion of coal; production of chlorine and cement factories also release mercury. The toxicity of mercury is strongly dependent on the types of chemical compounds it is in. The main problem is that in nature, many mercury compounds are converted into particularly dangerous methylmercury, which is concentrated in the food chain and contaminates marine animals in particular. People who regularly eat contaminated fish suffer from complaints such as headaches, pain in the limbs, heart, circulatory, and autoimmune diseases, paralysis, and blindness. Heavy chronic poisoning is deadly.
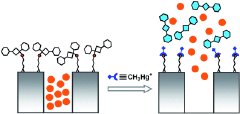
A team headed by Ramón Martínez-Máñez at the Polytechnical University of Valencia (Spain) and Knut Rurack at the Federal Institute of Materials Research and Testing (Berlin, Germany) has now developed a rapid test for the detection of this toxin based on the fact that mercury compounds are crazy about sulfur atoms. The researchers put dye molecules into the channels of an articifical porous mineral. On the surface of the mineral, they attached sulfur-containing groups, which in turn are attached to organic molecules that are so bulky that they cover the pores of the mineral like lids, keeping the dye inside. If a methylmercury-containing sample is added, it latches onto the sulfur-containing groups and splits off the "lids" of the pores. This opens the pores and releases the dye.
The main advantage of this approach is its amplification effect: only a few methylmercury particles are enough to release a large number of dye molecules. This allows for simple visual detection of trace concentrations of the toxin, even in complex biological samples. Another advantage is that the test is highly selective for methylmercury; water-soluble inorganic mercury compounds are excluded, so they do not result in a coloration.
The researchers imagine a kind of test strip that can simply be dipped into a prepared sample, of fish for example, to determine if it is contaminated with methylmercury.