A new plastic film, for which a patent has been applied, will protect BASF's pharmaceutical excipient polyvinylpyrrolidone (PVP) in future even better against the penetration of atmospheric oxygen and thus against oxidation. "With this, BASF will be able to offer its customers in the pharmaceutical industry an even higher product quality and, as a result, improved patient safety," says Dr. Boris Jenniches from BASF's global Business Management PVP.
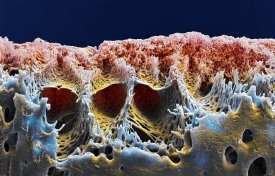 BASF is developing components for polymer membranes used for micro and ultra filtration. Ultra filtration membranes cleanse drinking water of bacteria and pathogens as well as of their decomposition products and viruses. One of the key components is polyvinylpyrrolidone (PVP). It forms the necessary pore structure in the pictured hollow fiber membrane.
BASF is developing components for polymer membranes used for micro and ultra filtration. Ultra filtration membranes cleanse drinking water of bacteria and pathogens as well as of their decomposition products and viruses. One of the key components is polyvinylpyrrolidone (PVP). It forms the necessary pore structure in the pictured hollow fiber membrane.
After all, the very highest quality standards are required for pharmaceutical applications. BASF's products, processes and facilities even exceed the strict international quality standards of the pharmaceutical industry. Consequently, the United States Pharmacopeia (USP), an independent institute, certified the PVP produced by BASF. The USP checked not only compliance with the requirements of current Good Manufacturing Practice (cGMP), but also the quality and the documentation of production control and quality control. At the same time, the PVP marketed by BASF was also certified for the European market in a wide-ranging GMP audit. "Awareness of the quality of pharmaceutical ingredients has been growing steadily. BASF guarantees a consistently high product quality and in this way contributes to the success of its pharmaceutical customers," says Jenniches.
Under the registered trade name Kollidon, PVP is used in tablets as a binding agent and disintegrant. It makes tablets a true high-tech product: as a binding agent it enables individual active ingredients of a tablet to form a homogenous entity and as a disintegrant it ensures that tablets break up in liquid and release the active ingredient quickly. A large proportion of the polyvinylpyrrolidones made by BASF goes to the pharmaceutical sector.
PVP is a product that has been a success story for a long time now. About 70 years ago, towards the end of 1938 and at the start of 1939 to be exact, the chemist Walter Reppe, working in a BASF laboratory in Ludwigshafen, used acetylene and pyrrolidone to produce a new monomer called vinylpyrrolidone, which in turn can be transformed into the polymer polyvinylpyrrolidone. On January 1, 1939 Reppe's method was patented. Quickly it became clear that the BASF researcher had discovered a veritable all-rounder: PVP is soluble in water, but it can also absorb large quantities of water; it is non-irritant to the skin and does not pose a health hazard; it is temperature-resistant, pH-stable, nonionic and colorless. Due to these varied features PVP can be used for a wide range of applications.
PVP is used in various BASF product lines, including those for the cosmetic, detergent and food industries. Luviskol, for example, as a component of hair gels and hair sprays, has been setting hair for over 50 years. Divergan is used in the filtration of beer and in the treatment of wine, ensuring that the drinks remain clear for longer.
PVP products marketed under the Luvitec brand are used in completely different technical applications. For example, Luvitec plays an important role in the manufacture of dialysis and water filtration membranes. In glue sticks it provides the right amount of adhesion. Because of its very varied properties, even today new applications are still being found for PVP, so the product will continue in future to have a firm place in the BASF portfolio.
The starting product for PVP, namely N-vinylpyrrolidone (NVP), has been produced since 1939 in Ludwigshafen and since 1992 also at BASF's Geismar site in the United States.