Scientists at the Hebrew University of Jerusalem and the University of Barcelona have reported in the journal ‘Nature Chemistry’ that the interactions between carbon-hydrogen groups, which are common in organic compounds, are very strong under certain conditions.
Scientists studied the polyhedranes created by carbon atoms adjoining hydrogen. These materials have the potential to produce stable crystal forms with up to 400 ºC of melting temperatures.
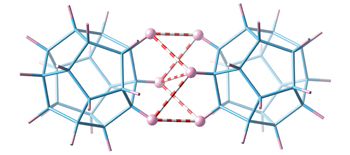 Intermolecular non-polar H···H interactions between polyhedrane molecules.
Intermolecular non-polar H···H interactions between polyhedrane molecules.
Santiago Àlvarez, a scientist from the Institute of Theoretical and Computational Chemistry and professor of the Department of Inorganic Chemistry at the University of Barcelona, stated that polyhedranes demonstrate very strong hydrogen interactions because they fulfill numerous chemical conditions. The scientists observed that carbon atom embracing the hydrogen is linked to a huge skeleton form comprising more number of carbon atoms, which accounts for the strong interaction, he said.
The research team conducted an organized computational investigation of homopolar hydrogen bonds. The study revealed that intermolecular interactions were stronger when the polyhedrane structure is flatter. The scientists also noticed that the spherical structure of polyhedrane makes interactions with adjoining molecules in numerous directions. Àlvarez said that these results describe the stronger cohesive forces in polyhedranes, which need high temperatures to raze their 3D structure to convert into a liquid form.
Àlvarez further said that these kinds of interactions are common in the molecular chemistry of coordination, organometallic and organic compounds. The research team will reassess the earlier studies like the relative stabilities of varied crystal forms in a single compound. This is a major breakthrough in the development of synthetic compounds, especially for the pharmaceutical market because each structure of polymorph demonstrates varied pharmacological characteristics. The industrial patents encompass only one type of these polymorphs, enabling other new forms of patents.