A team of researchers from the University of South Carolina (USC), Stanford University and Yonsei University has conducted a study on one of the aluminosilicate minerals, natrolite, which can open up new avenues for nuclear waste processing.
 Pressure surprisingly opens up the nanopores in a mineral.
Pressure surprisingly opens up the nanopores in a mineral.
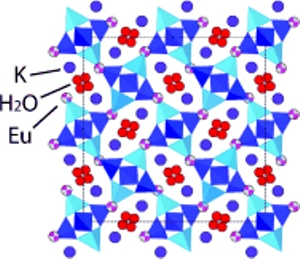
As a part of the research, they applied pressure on a porous solid and made its cavities expand, thus allowing entry and capture of europium (Eu3+) ions. Aluminosilicate minerals are referred as zeolites, which is made of tiny, regularly spaced pores. These minerals are available in over hundred different varieties and the composition of each one verifies the size of the cavity and the types of ions and molecules that can be retained or expelled. When mixed with a solution that contains a combination of ions, zeolites have an ability to selectively retain ions that can fit inside the pores.
Researchers used a stimulus, pressure, to control the size of the cavity within the zeolite. They were able to manage influencing of trivalent Eu3+ ions to enable replacement with K+ ions within the nanoscale cavities of the natrolite. Later, they captured the immobilized ions after removing the pressure. The team applied more than 1 GPa pressure in a diamond-anvil cell that led to greater expansion of cavities within the material. This allowed migration of larger ions within the pores and they get trapped, once the pressure is released. The study has demonstrated that it is possible to replace 90% of K+ ions with Eu3+ ions.
Thomas Vogt, a professor in the department of chemistry and biochemistry at the USC’s College of Arts and Sciences, expects that the study on the performance of natrolite under pressure can provide insight for scientists performing research on the earth's crust. The study results can also induce interest in several areas, including fracking, tectonics and mineralogy.